the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The youngest occurrence of embolomeres (Tetrapoda: Anthracosauria) from the Sunjiagou Formation (Lopingian, Permian) of North China
Jun Liu
Embolomeri were semiaquatic predators prevalent in the Carboniferous, with only two species from the early Permian (Cisuralian). A new embolomere, Seroherpeton yangquanensis gen. et sp. nov. (Zoobank Registration number: urn:lsid:zoobank.org:act:790BEB94-C2CC-4EA4-BE96-2A1BC4AED748, registration: 23 November 2020), is named based on a partial right upper jaw and palate from the Sunjiagou Formation of Yangquan, Shanxi, China, and is late Wuchiapingian (late Permian) in age. It is the youngest embolomere known to date and the only embolomere reported from North China Block. Its phylogenetic position within Embolomeri is confirmed by the strongly developed descending flange on the quadrate ramus of the pterygoid. The new taxon is unique among embolomeres by features like a partial coverage of a denticle shagreens on the pterygoid; presence of a cylindrical shaft on the pterygoid, and two pairs of very large ectopterygoid tusks. Phylogenetic analysis shows Seroherpeton as being the sister group of a clade consisting of Proterogyrinus, Archeria, and Pholiderpeton. We hypothesize that the dispersal and decline of the embolomeres from Carboniferous to late Permian (Lopingian) is related to the climate changes, especially aridification, of the paleotropical regions.
- Article
(3935 KB) -
Supplement
(455 KB) - BibTeX
- EndNote
Embolomeri are a monophyletic group of large crocodile-like, semiaquatic predators, prevalent in the Carboniferous and early Permian (Cisuralian) (Panchen, 1970; Smithson, 2000; Carroll, 2009; Clack, 2012). The clade is generally considered to be a stem member of the Reptiliomorpha, taxa that are more closely related to amniotes than to lissamphibians (Ruta et al., 2003; Vallin and Laurin, 2004; Ruta and Coates, 2007; Clack and Klembara, 2009; Klembara et al., 2014; Marjanovic and Laurin, 2019). Its relationship with the Permian–Triassic Chroniosuchia is still uncertain, but it is generally accepted that Chroniosuchia are not a subclade of the Embolomeri (Carroll, 2009; Clack and Klembara, 2009; Klembara et al., 2010; Schoch et al., 2010; Clack, 2012; Arbez et al., 2018; Marjanovic and Laurin, 2019). The distribution and diversity of Embolomere are closely related to the paleogeography and climate changes of the late Paleozoic. The earliest members of the group started to appear in the Late Mississippian, exemplified by Proterogyrinus, which was collected from both Scotland and USA (Holmes, 1984; Clack and Smithson, 2020). The Pennsylvanian, especially the Bashkirian–Moscovian, was when the embolomeres were the most diversified, evidenced by the well-documented specimens of Anthracosaurus and Pholiderpeton (Eogyrinus) and the less well-known Calligenethlon, Leptophractus, Neopteroplax, Pteroplax, Carbonoherpeton, and Palaeoherpeton (Clack, 1987, 2012; Cope, 1873; Holmes and Carroll, 2010; Panchen, 1964, 1972, 1977). All these animals were distributed in the swamp forests in Euramerica, then positioned in the tropics near the Equator, where the weather was warm and humid, a favorable environment for the embolomeres (Clack, 2012). Coming into the Permian, embolomeres experienced a dramatic decline in diversity, with only two taxa reported from the early Permian. One is Acheria, represented by abundant material from Texas and Oklahoma of North America (Holmes, 1989), and the other is the much less-known Aversor, presumably an eogyrinid, from the Kungurian Inta Formation (∼ 273 Ma) of Russia (Gubin, 1985; Steyer, 2000). The decline of the embolomeres was probably related to the gradual aridification of Euramerica during the Permian (Roscher and Schneider, 2006; Roscher et al., 2011; Bernardi et al., 2017), which resulted in the decrease of suitable habitats.
Here we report a new embolomere taxon from the late Permian (Lopingian) of Yangquan, Shanxi Province, China. The fossil was collected from the red mudstone of the basal layer of the Sunjiagou Formation (Fig. 1). The new taxon expands the chronological and geographical ranges of embolomeres and bears important implications on the late Paleozoic paleogeography and climate (Abbreviations: YQZY, Yuanquan Land Resources).
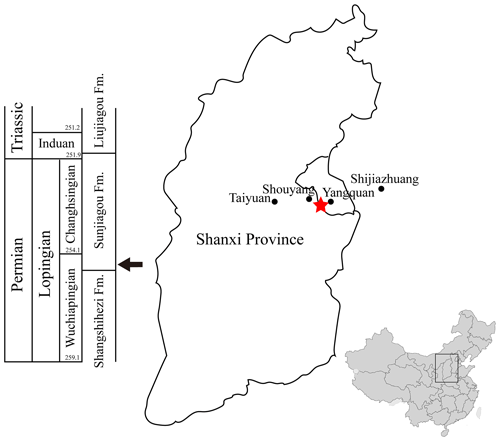
Figure 1Locality and horizon of Seroherpeton yangquanensis. The red star marks the locality of the holotype, near the border of Yangquan city and Shouyang County. The arrow at the bottom of the Sunjiagou Formation points to the horizon of the holotype. The map of China was modified from wikipedia.org.
Specimen. The specimen was collected from Cuifeng Mountain, Yangquan by a local (Bai Zhi-jun) during the 2010s and curated under YQZY. It was a partial palate and upper jaw from the red mudstone of the late Permian Sunjiagou Formation.
Phylogeny. We used the data matrix of Holmes and Carroll (2010), which was modified from Clack and Klembara (2009). We added three new characters based on the morphology of the pterygoid (see Appendix). The new matrix resulted in 10 taxa coded for 330 morphological characters. All coding followed Holmes and Carroll (2010) except for the newly added Seroherpeton. Caerorhachis was set as the outgroup. The parsimony analysis was performed in POY 5.1.1 (Wheeler et al., 2015) under the branch-and-bound algorithm.
-
Tetrapoda Jaekel, 1909
-
Reptiliomorpha Laurin, 2001
-
Anthracosauria Säve-Söderbergh, 1934
-
Embolomeri Cope, 1885
-
Seroherpeton yangquanensis gen. et sp. nov.
Entomology
Sero means “late” in Latin; herpeton means “crawling animal”. Yangquan is the city where the fossil was collected.
Holotype
YQZY JZ 1, a partially preserved right upper jaw and palate.
Locality and horizon
Yangquan, Shanxi Province. Basal layer of the Sunjiagou Formation (Wuchiapingian) (Liu, 2018) (Fig. 1).
Diagnosis
Large embolomere characterized by the combination of the following derived characters: posterior end of maxilla contacting pterygoid; ectopterygoid not bordering subtemporal fossa; two pairs of large ectopterygoid tusks; denticle shagreens partially covering the palatal ramus of pterygoid; presence of a cylindrical shaft along the quadrate ramus of pterygoid; descending flange of the quadrate ramus of pterygoid extending slightly below cheek.
Description
The holotype is a partial right upper jaw and palate, with incomplete preservation of the maxilla, ectopterygoid, pterygoid and quadrate. The total anterior–posterior length is 218 mm, the width at the widest point is 65 mm, and the depth is 95 mm. Estimated from the skull proportions of previously reported embolomeres (Anthracosaurus, Pholiderpeton, etc.), the skull length of Seroherpeton yangquanensis can easily be longer than 400 mm.
Maxilla
We interpret the holotype as having the maxilla preserved. Although the maxilla and ectopterygoid are pressed very close to each other, making the two superficially look like one bone, the suture line can be traced in several places from the dorsal and ventral view (Figs. 2, 3). Most posteriorly the suture line is not obvious, suggesting a possible partial fusion. The labial surface lacks a flat articulation surface for the overlapping of another bone, which indicates that this is the maxilla rather than the labial lamina of the ectopterygoid. It is smooth anteriorly. Starting from where the last ectopterygoid tusk lies, the maxilla bulges slightly laterally and bears pitted ornamentation, probably for muscle attachment (Fig. 2a, b). No traces of lateral line system can be found. The lingual side of the maxilla is narrow and bears teeth. The posterior border of the maxilla is intact. It is thickened to form a triangular medial process that abuts the pterygoid (Figs. 2c, d, 3), preventing the ectopterygoid from bordering the subtemporal fossa. Dorsally the maxilla is narrow, overlapping the ectopterygoid laterally. A flat and smooth area is present on the posteromedial side of the maxilla, probably as an insertion surface for the processeus alaris of the jugal (Fig. 2c, d).
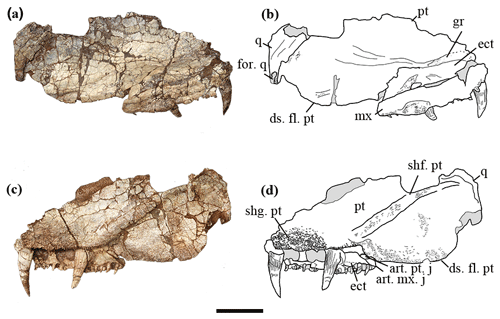
Figure 2The holotype of Seroherpeton yangquanensis: photo (a) and line drawing (b) in lateral view; photo (c) and line drawing (d) in medial view. Scale bar equals 50 mm. Abbreviations: art. mx. j: articulation of maxilla for the processeus alaris of the jugal; art. pt. j: articulation of pterygoid for the processeus alaris of the jugal; ds. fl. pt: descending flange of pterygoid; ect: ectopterygoid; for. q: quadrate foramen; gr: groove on pterygoid; mx: maxilla; n. for.: nerve foramen; pt: pterygoid; q: quadrate; shf. pt: shaft on pterygoid; shg. pt: denticle shagreen on pterygoid; tor. tr: torus transiliens.
Ectopterygoid
The ectopterygoid is partially preserved as an elongated trapezoid bone, missing the anterior margin. Two pairs of large tusks are present, separated by a narrow and grooved area 10 mm long (Fig. 3). The smooth area between the last tusk and the posterior border is 15 mm long. Dorsally, the ectopterygoid articulates with the pterygoid in a zigzag pattern (Fig. 2a, b). On the dorsal surface at the position where the last tusk lies, there is a small rounded concavity.
Pterygoid
The right pterygoid is partially preserved, missing the anterior part of the palatal ramus that articulates with the palatine and vomer, the dorsalmost part of the quadrate ramus that articulates with the epipterygoid, and the posteroventral extremity of the quadrate ramus.
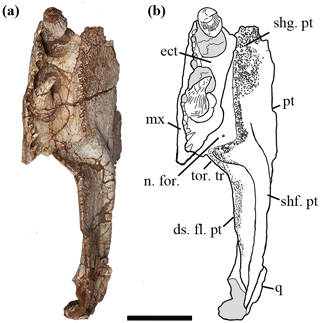
Figure 3Holotype of Seroherpeton yangquanensis: photo (a) and line drawing (b) in ventral view. Scale bar equals 50 mm. For abbreviations see Fig. 2.
Laterally the palatal shelf is horizontal, but medially it twists to form an “inner wall” with an angle of about 45∘. No articulation surface can be distinguished on the medial surface of the shelf, indicating no medial contact with its counterpart at least at the level of the ectopterygoid. Whether the two pterygoids met along the midline more anteriorly cannot be determined from the specimen. Judging from the overall straight border and orientation of the medial wall, there is little room for an interpterygoid vacuity, excluding the new specimen from being a temnospondyl. A narrow and shallow excavation is present on the dorsal surface of the pterygoid anteriorly (Fig. 2b).
Denticles partially cover the ventral surface of the palatal ramus. This denticulated area is separated from the pterygoid–ectopterygoid suture by a narrow strip of smooth bone. The suture between the ectopterygoid and pterygoid is more or less straight anteriorly, but posteriorly the pterygoid wraps around the ectopterygoid to abut the maxilla (Fig. 3). Close to this contact, a smooth area of the pterygoid is present at a slightly lower level than the ectopterygoid surface (Fig. 2c, d), which may represent an articulation surface for the processeus alaris of the jugal. Together with the insertion point of the jugal suggested on the maxilla, the ectopterygoid must have been excluded from bordering the subtemporal fossa. A nerve foramen can be observed on the postero-ventral surface of the smooth area of the palatal ramus (Fig. 3). Right behind where the denticle ridge curves, an oval concave notch about 25 mm × 15 mm is present, with shallow pitted ornamentation (Figs. 3, 4). The lateral edge to the notch is covered with extensive ornamentation and is homologous with what was mentioned in Holmes (1989) as the torus transiliens in Archeria, probably a housing point for the cartilago transiliens for the attachment of the musculus pterygoideus muscles (Witzmann and Werneburg, 2017). The distinct transverse process as seen in Chroniosuchia (Klembara et al., 2010; Arbez et al., 2018) is not developed in Seroherpeton, excluding the latter from the chroniosuchians.
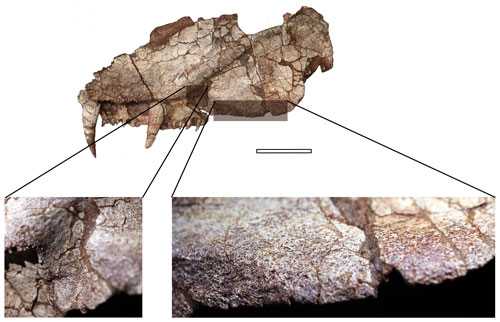
Figure 4Detailed look on the ornamentation pattern of the notch and ventromedial edge of the descending flange of the pterygoid. Scale bar equals 50 mm. The zoomed-in photos on the bottom are not to scale.
The medial surface of the pterygoid bears a long and distinctive cylindrical shaft running postero-dorsally from the transition point between the palatal ramus and quadrate ramus. It tapers towards the dorsal part of the quadrate (Figs. 2c, d, 3). The shaft bears fine pits that probably indicate muscle attachments (Fig. 2c, d).
Ventral to the shaft, the quadrate ramus of the pterygoid forms a deep descending flange with a rounded and convex ventral border (Figs. 2, 3). A similar descending flange has been described in other embolomeres (e.g., Proterogyrinus, Archeria). It extends below the ventral edge of the cheek. Unlike Anthracosaurus, the quadrate ramus of the pterygoid in this new taxon does not extend laterally beyond the palatal tusks to produce laterally swollen cheeks; rather the skull is a more or less triangular in dorsal profile as in Pholiderpeton and Proterogyrinus. The lateral surface of the quadrate ramus lacks ornamentation. A long but shallow depression is present with the long axis running horizontally near the ventral edge (Fig. 2a, b). The ventromedial border of the descending flange bears fine ornamentation and shallow pits, indicating extensive muscle attachment for M. pterygoideus muscles (Figs. 2c, d, 3, 4). Proportionally, the subtemporal fossa is rather large, indicating massive adductor muscles for a powerful bite. The dorsal surface of the pterygoid where it meets the epipterygoid is not preserved, so the morphology is uncertain. Posteriorly, the pterygoid is abutted by the quadrate laterally, but the ventral edges of neither bones are preserved.
Quadrate
The quadrate is a thin plate of bone, overlapping the pterygoid posterolaterally (Figs. 2, 3). The posteroventral portion of the quadrate is broken off, so the articular condyle is not preserved. Dorsally, the quadrate is thickened and expanded to form a dorsal flange over the pterygoid. A concavity can be seen dorsally, probably for articulation with the squamosal (Fig. 2a, b). On the lateral surface toward the ventral side, a horizontal groove, although incompletely preserved, is clearly present (Fig. 2a, b). This may represent the quadrate foramen between it and the quadratojugal.
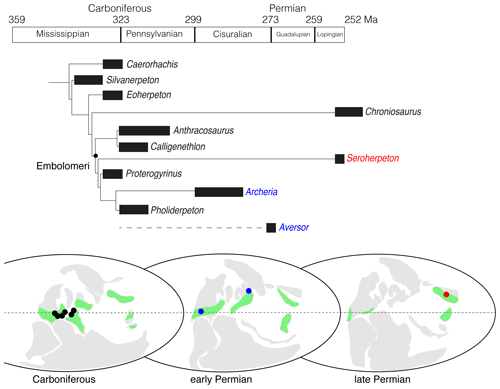
Figure 5Phylogeny and biogeography of embolomeres. The cladogram is calibrated based on the ages of the fossils. Carboniferous localities include northern England (Anthracosaurus, Pholiderpeton, and Pteroplax), Scotland (Anthracosaurus, Palaeoherpeton, Pholiderpeton, and Proterogyrinus), Joggins (Calligenethlon), and Florence (Carbonoherpeton) of Nova Scotia, Ohio (Leptophractus and Neopteroplax), and West Virginia (Proterogyrinus), USA; early Permian localities include Texas and Oklahoma, USA (Archeria), and Inta, Russia (Aversor); late Permian locality includes Shanxi, China (Seroherpeton). The green color represents the estimated range of tropical forests. All ages and localities except for Seroherpeton come from the literature (Clack, 1987, 2012; Cope, 1873; Gubin, 1985; Holmes, 1984, 1989; Holmes and Carroll, 2010; Panchen, 1964, 1977). The paleographic maps were modified from Tabor and Poulsen (2008, fig. 3).
Dentition
The holotype preserves the maxillary teeth, ectopterygoid tusks, and denticle shagreens on the pterygoid.
A total of 22 tooth positions (15 teeth and 7 alveoli) are preserved on the right maxilla. Two additional small teeth are preserved lateral to the main teeth. Between the anterior 9 teeth and the posterior 13 teeth, at the level of the last ectopterygoid tusk, lies a diastema of 12 mm long. The last four teeth are slightly larger than the anterior teeth. The crown of the second-to-last tooth measures 12 mm high, and the crown of the first preserved tooth measures 8 mm high. The tooth is conical and slightly recurved toward the tip with longitudinal grooves at the base of the crown, most resembling that of Pteroplax as illustrated by Clack (1987, fig. 8f, pp. 29), but differing from that of Pholiderpeton or Eogyrinus, in which the tooth barely tapers and bears a prominent anterior crest, reminiscent of the chisel-shaped tooth in Archeria (Clack, 1987, fig. 8a–e., pp. 29).
The ectopterygoid bears two pairs of large tusks (each represented by a large tusk and a companion replacement alveolus). The anterior margin of the ectopterygoid is not preserved, but based on the shape, position and proportion of the ectopterygoid, it is unlikely that there were more ectopterygoid tusks. The shape of the tusk resembles that of the maxillary tooth, with a conical and recurved shape and presence of longitudinal grooves. The anterior tusk measures 50 mm long and a basal diameter of 19 mm, and the posterior tusk measures 42 mm long and a basal diameter of 16 mm.
The third component of dentition is on the pterygoid. On the ventral side of the palatal ramus, denticle shagreens cover the more lateral part and gradually decrease in size and density towards the dorsomedial wall (Figs. 2c, d, 3). The denticles along the lateral edge are larger than the more medial ones and form a prominent toothed ridge parallel with the marginal teeth and the palatal tusks (Fig. 3). The ridge curves laterally towards the maxilla posteriorly. The largest denticles along the ridge measure 3 mm long.
The holotype of Seroherpeton is a partial palate and upper jaw found from the late Permian (Lopingian) of North China. At first glance, its morphology is similar to tetrapodomorphs such as Eusthenopteron, especially with its large palatal tusks, the denticles on the palatal ramus of the pterygoid, and the oblique shaft on the pterygoid. However, the resemblance is largely due to the conservative nature of the palate throughout the evolution of tetrapodomorphs and early tetrapods (Kimmel et al., 2009). Large paired ectopterygoid tusks are common among lobe-finned fish (Jarvik, 1980; Clack, 2012). They were lost in the earliest tetrapods such as Ichthyostega and Acanthostega but were re-acquired in many later tetrapod clades such as temnospondyls (e.g., Eryops, Phonerpeton), baphetids (e.g., Megalocephalus, Loxomma), and embolomeres (e.g., Anthracosaurus) as independent derived conditions. Among embolomeres, the size of the tusk varies across taxa. With an estimated skull length of around 400 mm, the large tusk of the Seroherpeton is comparable to that of similar-sized Anthracosaurus, an assumed top predator of its time. Anthracosaurus has an ectopterygoid tusk of 45 mm long and a basal diameter of 15 mm (Panchen, 1977), slightly larger than the posterior tusk but smaller than the anterior tusk of the new taxon. Pholiderpeton of similar size, on the other hand, has much smaller tusks, with the largest one of only 29 mm long and a basal diameter of 7 mm (Clack, 1987). In both tetrapodomorphs (such as Eusthenopteron) and the new taxon, a row of teeth is very closely present lateral to the paired ectopterygoid tusks. However, in tetrapodomorphs, the teeth are on ectopterygoid, whereas in the new taxon, we interpret the teeth as maxillary teeth.
The presence of denticles on the pterygoid is another common character seen in various lobe-finned fish and tetrapods as a plesiomorphic condition. Among embolomeres, the degrees of denticle coverage vary. Anthracosaurus has a complete smooth pterygoid surface with no coverage of denticles (Panchen, 1977), whereas eogyrinids and proterogyrinids have extensive coverage of denticles over the ventral surface of the pterygoid (Panchen, 1972; Holmes, 1984). Seroherpeton has the denticles covering only part of the palatal ramus of the pterygoid, a presumably intermediate condition.
The presence of a prominent oblique shaft on the pterygoid of Seroherpeton recalls the similar condition in tetrapodomorph Eusthenopteron (Jarvik, 1980) and Medoevia (Lebedev, 1995). Jarvik (1980) interpreted the function of the shaft as providing a resting place for the ceratohyal and a possible water passage through the external gill in Eusthenopteron. None of the other known embolomeres has such a shaft. It is certainly a curious condition in the new taxon and may represent an autapomorphic condition for Seroherpeton among embolomeres.
Embolomeri is a monophyletic group characterized by synapomorphies such as the presence of the tabular horn, the absence of post temporal fossa, and the presence of a large Meckelian fenestra in the mandible (Smithson, 1985, 2000); its monophyly was supported in all recent phylogenies (Ruta et al., 2003; Ruta and Coates, 2007; Clack and Klembara, 2009; Arbez et al., 2018; Marjanovic and Laurin, 2019). Seroherpeton does not preserve the abovementioned synapomorphies but nevertheless has a derived pterygoid morphology that indicates its embolomere affinity. The muscle scars on the notch behind the pterygoid denticles and the medioventral border of the descending flange (Fig. 4) indicate extensive muscle attachment of M. pterygoideus (Holmes, 1989; Witzmann and Werneburg, 2017). Holmes (1989), in describing Archeria, considered the strongly developed descending flange and cartilago–torus transiliens as shared derived characters of embolomeres, and possibly their form and function are homologous with those of basal reptiliomorphs. A similar system was also known in Paleoherpeton (Panchen, 1964), Pholiderpeton (Clack, 1987), and a new possible embolomere from the Mississippian of Scotland (Clack and Smithson, 2020). This muscle attachment pattern is clearly missing in tetrapodomorphs (e.g., Eusthenopteron), in which the pterygoid lacks such an ornamented notch and has a smooth ventral edge of the quadrate ramus (Jarvik, 1980; Lebedev, 1995).
We ran a parsimony analysis to determine the phylogenetic position of Seroherpeton within embolomeres. It resulted in one most parsimonious tree with 199 steps (Fig. 5). The major difference between our tree and that of Holmes and Carroll (2010) is that Chroniosaurus lies more towards the crown than Eoherpeton in the new tree. Seroherpeton lies more towards the crown than Anthracosaurus + Calligenethlon, and forms the sister group of the clade consisting of Proterogyrinus, Pholiderpeton, and Archeria.
This result posts an interesting question of when and how the lineage leading to Seroherpeton occurred in the North China Block (NCB). Both the locality and age of the new taxon lie outside the previously known distribution of the clade. Embolomeri is predominantly a Euramerican group with the largest diversity in the Carboniferous. Sediments in NCB during the Carboniferous are mostly marine, and the block is distantly separated from Euramerica as an isolated island (Huang et al., 2018). It is therefore unlikely that embolomeres were distributed in NCB during the Pennsylvanian. Abundant fossils of Archeria in North America showed that although the clade as a whole were not as diversified in species number, it was still a major component in the Euramerican fauna during the early Permian. Although less studied, the appearance of Aversor, presumably an eogyrinid, in the Kungurian of Russia (Gubin, 1985) showed that the embolomeres had migrated to a slightly higher latitude by the beginning of the Guadalupian. The lineage leading to Seroherpeton may have existed as a “ghost lineage” for a long time and dispersed to NCB through Siberia/Tarim in the late Permian, depending on the availability of land bridges. Different studies have predicted different scenarios of land connection between NCB and other blocks in the late Permian, but Liu et al. (2020) showed that the connection must have existed since at least 256 Ma (evidenced by fossils found from the Shangshihezi Formation in NCB). NCB was warm and humid throughout the Permian (Tabor and Poulsen, 2008; Bernardi et al., 2017), providing embolomeres with favorable environments. In the meantime, with Euramerica gradually becoming more arid during the Permian (Roscher and Schneider, 2006; Tabor and Poulsen, 2008; Roscher et al., 2011; Bernardi et al., 2017), suitable habitats for embolomeres such as humid tropical forests became more sporadic in Euramerica (Fig. 5). As noted in Bernardi et al. (2017), the low-latitude faunas in the Lopingian tend to preserve the last-surviving members of clades better known from earlier ages (such as captorhinids and temnospondyls), serving as a “natural museum”. The occurrence of Seroherpeton in NCB is another example of it (Fig. 5). Nevertheless, the discovery of Seroherpeton showed that the Embolomeri as a clade have survived through the middle Permian extinction (Olson's Extinction) and, with the right environments, might occur elsewhere during the late Permian.
Three new characters were added to the character list of Clack and Klembara (2009) [8] as follows:
-
328. Descending flange on the quadrate ramus of pterygoid: absent (0); present, but not extending below the ventral margin of the cheek (1); extending below the cheek margin.
-
329. Quadrate ramus of pterygoid: stays at more or less a straight line with the palatal tusks, resulting in a triangular skull shape (0); turn lateral to the palatal tusks, resulting in a more laterally expanded posterior region of the skull (1).
-
330. A cylindrical shaft on the quadrate ramus of pterygoid: absent (0); present (1).
Data (phylogenetic data matrix) can be found in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/fr-23-205-2020-supplement.
JC and JL designed and performed the research and wrote the manuscript.
The authors declare that they have no conflict of interest.
Bai Zhijun collected the specimen and provided it to our study. Fieldwork was co-performed by Yi Jian, Liu Yu-Feng, and Liu Yu-Dong. The fossil was carefully prepared by Fu Hua-Lin. We thank Zhu You-An and Yi Hong-Yu for helpful discussions.
This research has been supported by the Strategic Priority Research Program of Chinese Academy of Sciences (grant no. XDB26000000).
This paper was edited by Florian Witzmann and reviewed by Robert Holmes and Timothy Smithson.
Arbez, T., Sidor, C. A., and Steyer, J. S.: Laosuchus naga gen. et sp. nov., a new chroniosuchian from South-East Asia (Laos) with internal structures revealed by micro-CT scan and discussion of its palaeobiology, J. Syst. Palaeontol., 17, 1165–1182, https://doi.org/10.1080/14772019.2018.1504827, 2018.
Bernardi, M., Petti, F. M., Kustatscher, E., Franz, M., Hartkopf-Fröder, C., Labandeira, C. C., Wappler, T., van Konijnenburg-van Cittert, J. H. A., Peecook, B. R., and Angielczyk, K. D.: Late Permian (Lopingian) terrestrial ecosystems: A global comparison with new data from the low-latitude Bletterbach Biota, Earth-Sci. Rev., 175, 18–43, https://doi.org/10.1016/j.earscirev.2017.10.002, 2017.
Carroll, R. L.: The rise of amphibians: 365 million years of evolution, The Johns Hopkins University Press, Baltimore, 2009.
Clack, J. A.: Pholiderpeton scutigerum Huxley, an amphibian from the Yorkshire coal measures, Philos. T. R. Soc. Lond. B, 318, 1–107, https://doi.org/10.1098/rstb.1987.0082, 1987.
Clack, J. A.: Gaining ground: the origin and evolution of tetrapods, Indiana University Press, Bloomington, Indiana, 2012.
Clack, J. A. and Klembara, J.: An articulated specimen of Chroniosaurus dongusensis and the morphology and relationships of the chroniosuchids, Spec. Pap. Palaeontol., 81, 15–42, 2009.
Clack, J. A. and Smithson, T. R.: A new large embolomere from East Kirkton, Scot. J. Geol., 56, 153–158, 2020.
Cope, E. D.: On some new Batrachia and fishes from the coal measures of Linton, Ohio, P. Acad. Nat. Sci. Phila., 1873, 340–343, 1873.
Gubin, Y. M.: First anthracosaur from the Permian of the East European Platform, Paleontol. Zh., 3, 118–122, 1985.
Holmes, R.: The Carboniferous amphibian Proterogyrinus scheelei Romer, and the early evolution of tetrapods, Philos. T. R. Soc. Lond. B, 306, 431–524, https://doi.org/10.1098/rstb.1984.0103, 1984.
Holmes, R.: The skull and axial skeleton of the Lower Permian anthracosauroid amphibian Archeria crassidisca Cope, Palaeontographica, Abteilung A, Paläozoologie, Stratigraphie, 207, 161–206, 1989.
Holmes, R. and Carroll, R. L.: An articulated embolomere skeleton (Amphibia: Anthracosauria) from the Lower Pennsylvanian (Bashkirian) of Nova Scotia, Can. J. Earth Sci., 47, 209–219, https://doi.org/10.1139/e10-008, 2010.
Huang, B., Yan, Y., Piper, J. D., Zhang, D., Yi, Z., Yu, S., and Zhou, T.: Paleomagnetic constraints on the paleogeography of the East Asian blocks during Late Paleozoic and Early Mesozoic times, Earth-Sci. Rev., 186, 8–36, https://doi.org/10.1016/j.earscirev.2018.02.004, 2018.
Jarvik, E.: Basic Structure and Evolution of Vertebrates, Academic Press, London, 575 pp., 1980.
Kimmel, C. B., Sidlauskas, B., and Clack, J. A.: Linked morphological changes during palate evolution in early tetrapods, J. Anat., 215, 91–109, 2009.
Klembara, J., Clack, J. A., and Černansky, A.: The anatomy of palate of Chroniosaurus dongusensis (Chroniosuchia, Chroniosuchidae) from the Upper Permian of Russia, Palaeontology, 53, 1147–1153, https://doi.org/10.1111/j.1475-4983.2010.00999.x, 2010.
Klembara, J., Clack, J. A., Milner, A. R., and Ruta, M.: Cranial anatomy, ontogeny, and relationships of the Late Carboniferous tetrapod Gephyrostegus bohemicus Jaekel, 1902, J. Vertebr. Paleontol., 34, 774–792, https://doi.org/10.1080/02724634.2014.837055, 2014.
Lebedev, O. A.: Morphology of a new osteolepidid fish from Russia, Bull. Mus. Nat. Hist. Nat. C, 17, 287–341, 1995.
Liu, J.: New progress on the correlation of Chinese terrestrial Permo-Triassic strata, Vertebrat. Palasiatic., 56, 327–342, https://doi.org/10.19615/j.cnki.1000-3118.180709, 2018.
Liu, J., Yi, J., and Chen, J.-Y.: Constraining assembly time of some blocks on eastern margin of Pangea using Permo-Triassic non-marine tetrapod records, Earth-Sci. Rev., 207, 103215, https://doi.org/10.1016/j.earscirev.2020.103215, 2020.
Marjanovic, D. and Laurin, M.: Phylogeny of Paleozoic limbed vertebrates reassessed through revision and expansion of the largest published relevant data matrix, PeerJ, 6, e5565, https://doi.org/10.7717/peerj.5565, 2019.
Panchen, A. L.: The cranial anatomy of two Coal Measure anthracosaurs, Philos. T. R. Soc. Lond. B, 247, 593–636, https://doi.org/10.1098/rstb.1964.0006, 1964.
Panchen, A. L.: Teil 5a Anthracosauria. Handbuch der Paläoherpetologie, Teil 5A, Anthracosauria, Stuttgart, Fischer, Fischer, Stuttgart, 1970.
Panchen, A. L.: The skull and skeleton of Eogyrinus attheyi Watson (Amphibia: Labyrinthodontia), Philos. T. R. Soc. Lond. B, 263, 279–326, https://doi.org/10.1098/rstb.1972.0002, 1972.
Panchen, A. L.: On Anthracosaurus russelli Huxley (Amphibia: Labyrinthodontia) and the family Anthracosauridae, Philos. T. R. Soc. Lond. B, 279, 447–512, https://doi.org/10.1098/rstb.1977.0096, 1977.
Roscher, M. and Schneider, J. W.: Permo-Carboniferous climate: Early Pennsylvanian to Late Permian climate development of central Europe in a regional and global context, Geol. Soc. Lond. Spec. Publ., 265, 95–136, https://doi.org/10.1144/GSL.SP.2006.265.01.05, 2006.
Roscher, M., Stordal, F., and Svensen, H.: The effect of global warming and global cooling on the distribution of the latest Permian climate zones, Palaeogeogr. Palaeocl., 309, 186–200, https://doi.org/10.1016/j.palaeo.2011.05.042, 2011.
Ruta, M. and Coates, M. I.: Dates, nodes and character conflict: addressing the lissamphibian origin problem, J. Syst. Palaeontol., 5, 69–122, https://doi.org/10.1017/s1477201906002008, 2007.
Ruta, M., Coates, M. I., and Quicke, D. L.: Early tetrapod relationships revisited, Biol. Rev. Camb. Philos. Soc., 78, 251–345, https://doi.org/10.1017/s1464793102006103, 2003.
Schoch, R. R., Voigt, S., and Buchwitz, M.: A chroniosuchid from the Triassic of Kyrgyzstan and analysis of chroniosuchian relationships, Zool. J. Linn. Soc.-Lon., 160, 515–530, https://doi.org/10.1111/j.1096-3642.2009.00613.x, 2010.
Smithson, T.: The morphology and relationships of the Carboniferous amphibian Eoherpeton watsoni Panchen, Zool. J. Linn. Soc., 85, 317–410, 1985.
Smithson, T. R.: Anthracosaurs, in: Amphibian biology, edited by: Heatwole, H. and Carroll, R. L., Surrey Beatty and Sons, Chipping Norton, NSW 2170, 1053–1063, 2000.
Steyer, J. S.: Are European Paleozoic amphibians good stratigraphic markers?, B. Soc. Geol. Fr., 171, 127–135, 2000.
Tabor, N. J. and Poulsen, C. J.: Palaeoclimate across the Late Pennsylvanian–Early Permian tropical palaeolatitudes: a review of climate indicators, their distribution, and relation to palaeophysiographic climate factors, Palaeogeogr. Palaeocl., 268, 293–310, https://doi.org/10.1016/j.palaeo.2008.03.052, 2008.
Vallin, G. and Laurin, M.: Cranial morphology and affinities of Microbrachis, and a reappraisal of the phylogeny and lifestyle of the first amphibians, J. Vertebr. Paleontol., 24, 56–72, https://doi.org/10.1671/5.1, 2004.
Wheeler, W. C., Lucaroni, N., Hong, L., Crowley, L. M., and Varón, A.: POY version 5: phylogenetic analysis using dynamic homologies under multiple optimality criteria, Cladistics, 31, 189–196, https://doi.org/10.1111/cla.12083, 2015.
Witzmann, F. and Werneburg, I.: The palatal interpterygoid vacuities of temnospondyls and the implications for the associated eye-and jaw musculature, Anat. Rec., 300, 1240–1269, https://doi.org/10.1002/ar.23582, 2017.




