the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
†Cretolixon – a remarkable new genus of rhopalosomatid wasps (Hymenoptera: Vespoidea: Rhopalosomatidae) from chemically tested, mid-Cretaceous Burmese (Kachin) amber supports the monophyly of Rhopalosomatinae
Volker Lohrmann
Qi Zhang
Peter Michalik
Jeremy Blaschke
Patrick Müller
Laurent Jeanneau
Vincent Perrichot
Rhopalosomatidae, currently considered the sister group of the Vespidae, are an enigmatic family of aculeate wasps that originated in the Late Jurassic or Early Cretaceous. Despite their considerable age, very few fossils of the family have been reported – all of them in amber (Miocene Dominican, Miocene Mexican, and mid-Cretaceous Burmese ambers). Here we report a new mid-Cretaceous rhopalosomatid wasp, Cretolixon alatum Lohrmann, gen. et sp. nov., from Burmese (Kachin) amber. This new genus has a unique mixture of characters, some of which are only known from the recent brachypterous genus Olixon and others of which are known only from the recent macropterous genera. Thus, Cretolixon Lohrmann, gen. nov. not only provides further evidence for the monophyly of the family but also contributes evidence for the monophyly of the Rhopalosomatinae. Key characters of the family are discussed, and an updated checklist of the world genera and fossil species and occurrences of Rhopalosomatidae is provided. Additionally, a chemical analysis was performed for three of the newly reported fossils as well as for the amber piece containing the rhopalosomatid larva described by Lohrmann and Engel (2017) to ascertain their amber vs. copal nature and their affinities with each other and previously described Burmese amber.
Rhopalosomatidae, currently considered the sister group of the well-known Vespidae (Pilgrim et al., 2008; Branstetter et al., 2017), are an enigmatic family of aculeate wasps that originated in the Late Jurassic (Brady et al., 2009) or Early Cretaceous (Wilson et al., 2013; Branstetter et al., 2017). Notwithstanding their geological age or the species diversity of their putative sister, Rhopalosomatidae are a species-poor family, currently comprising less than a hundred recent species assigned to four genera (Aguiar et al., 2013). The majority of recent species occurs in the tropics and subtropics around the world (Townes, 1977). Based on current knowledge, members of the family are larval ectoparasitoids of crickets (e.g., Perkins, 1908; Hood, 1913; Gurney, 1953; Lohrmann et al., 2014; Miller et al., 2019) – a habit that has seemingly remained unchanged since the mid-Cretaceous (Lohrmann and Engel, 2017).
Within the family, two distinct morphological forms exist, which prompted Engel (2008) to split the family into two subfamilies – the Olixoninae Engel and Rhopalosomatinae Ashmead. The Olixoninae are solely represented by the highly apomorphic, brachypterous genus Olixon Cameron (1887), while the Rhopalosomatinae include the three remaining recent macropterous genera (Liosphex Townes, 1977; Paniscomima Enderlein, 1904; and Rhopalosoma Cresson, 1865). Engel also included the fossil genus Propalosoma in his Rhopalosomatinae, but this genus is now considered a formicoid wasp (Archibald et al., 2018). Unfortunately, Engel's subfamily division was not based on a phylogenetic analysis, and most diagnostic characters listed by him for the Rhopalosomatinae are most likely plesiomorphies for the family rather than derived characters for the subfamily. Nevertheless, Engel's classification is supported by earlier morphological and unpublished molecular analyses suggesting Olixon to be the sister to a monophylum comprising the recent macropterous genera (Brothers, 1999; Guidotti, 1999; Blaschke et al., unpublished results). Guidotti (1999) listed 22 potential synapomorphies for the Rhopalosomatinae, but for most of these it is not clear whether they have evolved in the stem of the family or the subfamily mainly due to (a) the highly derived morphology of Olixon, which is linked to the reduction of wings, and (b) the lack of stem group fossils elucidating the early evolution of the family.
In fact, very few fossils have been assigned to the family Rhopalosomatidae. True fossil rhopalosomatids (i.e., Eorhopalosoma gorgyra Engel, 2008; Eorhopalosoma lohrmanni Boudinot and Dungey, 2020; Rhopalosoma hispaniola Lohrmann (in Lohrmann et al., 2019); Rhopalosoma sp.; and an unassigned rhopalosomatid larva) have been described from mid-Cretaceous Burmese, Miocene Dominican, and Miocene Mexican ambers only (Engel, 2008; Lohrmann and Engel, 2017; Lohrmann et al., 2019; Boudinot and Dungey, 2020). In contrast all compression fossils described within the family (i.e., Mesorhopalosoma Darling (in Darling and Sharkey, 1990); Paleorhopalosoma Nel et al., 2010; and Propalosoma Dlussky and Rasnitsyn, 1999) are now considered misidentifications and do not belong to Rhopalosomatidae (Osten, 2007; Archibald et al., 2018) (Table 1).
Table 1Checklist of the world genera and fossil species and occurrences of Rhopalosomatidae with information on the species diversity and distributiona.
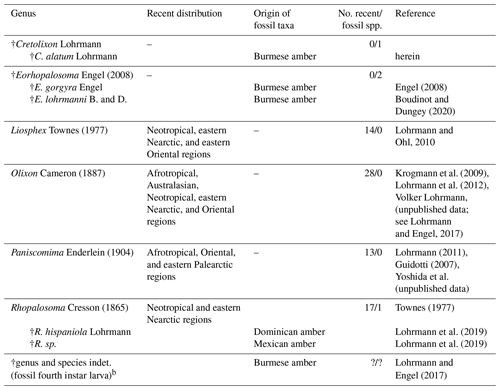
a Three additional fossil taxa, originally described within the family, have been transferred to other groups, i.e., Mesorhopalosoma Darling (in Darling and Sharkey, 1990) (mid-Cretaceous) and Paleorhopalosoma Nel et al., 2010 (Paleocene) have been transferred to sphecoid wasps (Osten, 2007; Archibald et al., 2018) and Propalosoma Dlussky and Rasnitsyn, 1999 (middle Eocene) to myrmeciine ants (Archibald et al., 2018). Furthermore, Grimaldi and Engel (2005) and Engel (2008) make mention of an additional fossil, Lithoserix williamsi Brown, 1986, which purportedly had been assigned to the family by Nel (1991). But in his publication Nel assigned the fossil as “Hymenoptera Vespina Vespomorpha (?), famille incertae sedis, nov. stat.” and makes no mention of the family Rhopalosomatidae. Actually, the species was placed within Ichneumonidae by Kasparyan and Rasnitsyn (1992). b The larva may or may not belong to one of the rhopalosomatid species already described from Burmese amber.
Here, we describe and illustrate an enigmatic new genus of rhopalosomatid wasps from mid-Cretaceous Burmese (Kachin) amber using four recently discovered specimens with a remarkable mix of characters. Based on our analyses, we discuss key characters of the family and provide an updated checklist of the world genera and fossil species of Rhopalosomatidae. In order to validate the origin of the specimens, we performed chemical analyses of three of the four amber pieces containing the investigated fossils.
2.1 Material studied
The new fossils have been compared with type material of different species representing all genera currently assigned to the family Rhopalosomatidae, i.e., Eorhopalosoma, Liosphex, Olixon, Paniscomima, and Rhopalosoma. Furthermore, more than 30 additional rhopalosomatid wasps in Burmese amber have been studied to investigate the morphological variation in these Cretaceous fossils. The material used and described in this study is deposited in the following institutions:
-
NIGP – Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (CAS), Nanjing, China
-
SNSB – Bayerische Staatssammlung für Paläontologie und Geologie, Staatliche Naturwissenschaftliche Sammlungen Bayerns, Munich, Germany
-
UMB – Übersee-Museum Bremen, Bremen, Germany
-
ZMB – Museum für Naturkunde, Berlin, Germany.
2.2 Origin and age of the amber
The four newly reported specimens are each preserved in a clear, yellowish piece of amber. Burmese amber originates from several mining sites in Kachin State (“Kachin amber”, ca. 98 Ma), Sagaing Region (“Hkamti amber”, ca. 110 Ma), and Magway Region (“Tilin amber”, ca. 72 Ma), but distinction between these deposits is rarely documented on the amber market (Zheng et al., 2018; Xing and Qiu, 2020). Miocene amber and even recent copal also apparently exist in the country, although with no known precise locality (Cockerell, 1922). Some pieces of these younger resins are sometimes offered by traders among bags of so-called “mid-Cretaceous Kachin amber” (Vincent Perrichot, personal observation, 2019), making their actual origin difficult to distinguish. The holotype MB.I 6500 studied herein was originally accessed by Sieghard Ellenberger (Kassel, Germany) in the early 2010s, directly from the Noije Bum site of the Hukawng Valley in Kachin State. It was then sold to the late Jens-Wilhelm Janzen (Seevetal, Germany) and eventually purchased by the ZMB. The three other fossils investigated herein were accessed no later than 2017 and supposedly originate from mines in the Hukawng Valley as well. Since one of our samples was obtained from an online auction (VL-Bu-04), we performed a chemical analysis to ascertain its amber vs. copal nature and its affinities with two of the other amber pieces. Chemical analyses confirmed the samples to be similar to the referenced Kachin amber (see results in Sect. 3.1 and the discussion below), thus from mines in the Hukawng Valley of Kachin State, northern Myanmar. The age of Kachin amber has been established as approximately 98 Ma based on radiometric dating of zircons (Shi et al., 2012), and bivalve borings and an ammonite found in an inclusion support an age dating to around the Albian–Cenomanian boundary (Smith and Ross, 2017; Yu et al., 2019). This period is generally referred to informally as the “mid-Cretaceous”.
2.3 Thermochemolysis–gas chromatography–mass spectrometry
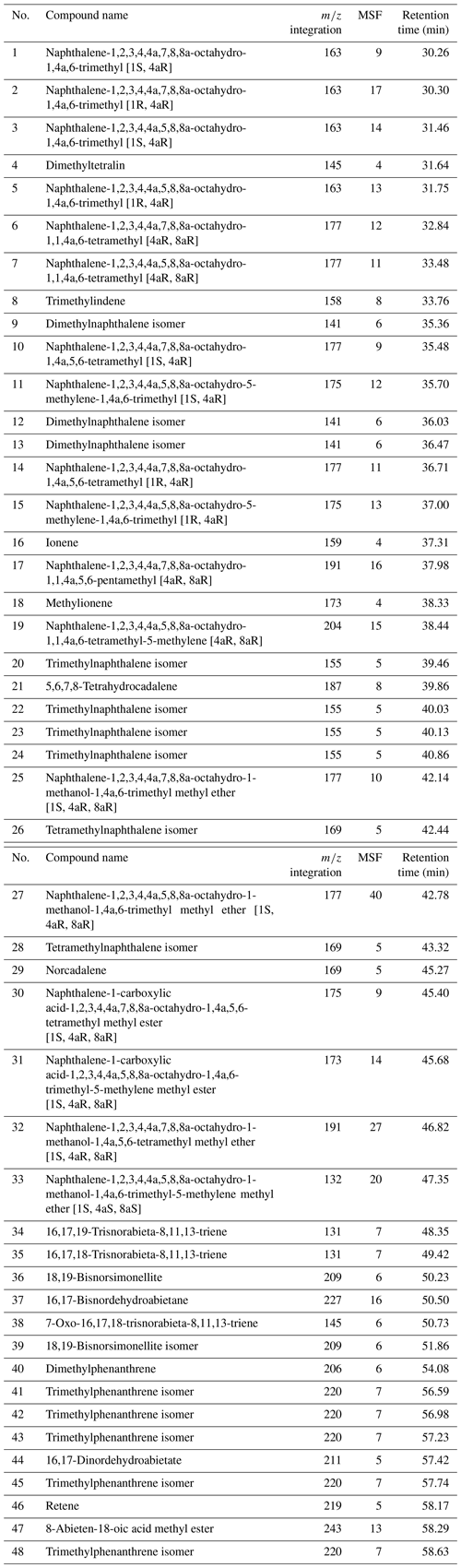
Amber pieces including three of the four new specimens (MB.I 6500, SNSB-BSPG 2020 XCIII 25, VL-Bu-04) and the rhopalosomatid larva published by Lohrmann and Engel (2017; here VL-Bu-10) were analyzed by thermally assisted hydrolysis and methylation (THM) also called thermochemolysis. A yellowish Baltic amber was analyzed as a reference to determine their chemical class. An additional amber piece containing a female paratype of the new species (NIGP173865) could not be analyzed for safety reasons (the amber surface was very close to the fossil inclusion, so the risk of damage during amber sampling was too high), but since the fossil it contains is conspecific with the three others we assume that all four pieces are of the same age and NIGP173865 cannot originate from the older Hkamti site or younger Tilin site.
Small portions of amber were crushed manually in an agate mortar. The powders (∼ 0.1 mg) were heated with TMAH (tetramethylammonium hydroxide) at 400 ∘C. THM was carried out using a Frontier Lab PY-2020iD pyrolyzer coupled with a Shimadzu GCMS-QP2010 Plus system operating with a split ratio of 100. Separation was achieved using a capillary column SLB-5MS (60 m × 0.25 mm inner diameter, 0.25 µm film thickness) with the carrier gas He with a flow of 1.1 mL min−1. The operating conditions were as follows: initial temperature held at 50 ∘C for 2 min and increased to 310 ∘C at a rate of 4 ∘C min−1 for 3 min. Individual compounds were identified based on comparisons of (i) mass spectrometry (MS) data with the NIST 2014 library and literature (Anderson, 1994, 1995; Dutta et al., 2011; Fischer et al., 2017) and (ii) retention time with the Baltic amber.
The relative distribution of identified compounds was determined by measuring the area of a specific fragment, denoted m∕z integration in Table A1. The peak area of the selected m∕z for each compound was integrated and corrected by a mass spectra factor (Table A1) calculated as the reciprocal of the proportion of the fragment used for the integration and the entire fragmentogram. The molecular ratios were calculated using those corrected areas, which allows an approximation of areas on the total ion chromatogram while preventing the integration of co-eluting compounds.
2.4 Preparation and study of fossils
All amber pieces were manually polished using a Buehler Metaserv 3000 polisher and Buehler CarbiMet silicon carbide papers to obtain flat surfaces for optimal observation and imaging of the insect inclusions.
The inclusions were studied with a Leica MZ12 and a Zeiss SteREO Discovery.V20 stereomicroscope. Measurements were taken with an ocular micrometer. However, due to the preservation in amber not all characters could be measured in a direct vertical view. Thus, measurements are approximations rather than exact numbers.
Extended-focus images were taken with a Leica DFC490 digital camera with a Leica Z16 APO system or with the BK PLUS Lab System (Dun, Inc.) with a Canon MP-E 65 mm lens mounted on a Canon 6D camera. Pictures were aligned and stacked using the software package Auto-Montage Essentials by Syncroscopy (Version 5.03.0061 ES) or Zerene Stacker under the PMax value.
2.5 Terminology
The general morphological terminology is adopted from Huber and Sharkey (1993) with additions from Mason (1986, 1990), and the following morphological abbreviations and modifications are used in the text: Cu2 is the section of fore wing cubitus separating the 2Cu and 2M (not closed) cells (Fig. 7). FI, FII, etc. are flagellomere I, flagellomere II, etc. IOD is the interocellar distance or the shortest distance between the lateral ocelli. LOD is the maximum diameter of a lateral ocellus. MOD is the maximum diameter of the median ocellus. M1 is the section of fore wing media separating the R and 1M cells (Fig. 7). M2 is the section of fore wing media separating the 1M and 1Rs cells (Fig. 7). OOD is the ocellocular distance or the shortest distance between the lateral ocellus and eye. Rs1 is the section of the fore wing radial sector separating the 1R1 and 1Rs cells (Fig. 7). Rs* is the section of the hind wing radial sector separating the R and R1 cells (Fig. 7). Tarsal fenestrae are sublateral, slightly transparent, sclerotized, less setose areas at the apex of the female tarsomeres in many macropterous Rhopalosomatidae. TL is the temple length or shortest distance between the posterior eye margin and occipital carina.
2.6 Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank (http://www.zoobank.org/, last access: 3 December 2020, date of registry: 5 December 2019), with the following LSID (reference): urn:lsid:zoobank.org:pub:3650519D-0470-42E2-97D7-A267658C0B4F. The electronic edition of this work has been archived and is available from the following digital repositories: Deutsche Nationalbibliothek, US Library of Congress, Portico, and CLOCKSS.
3.1 Chemical analysis
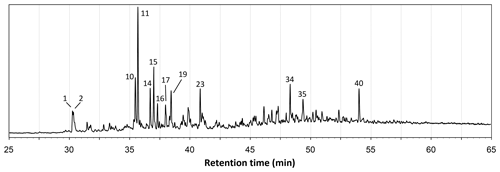
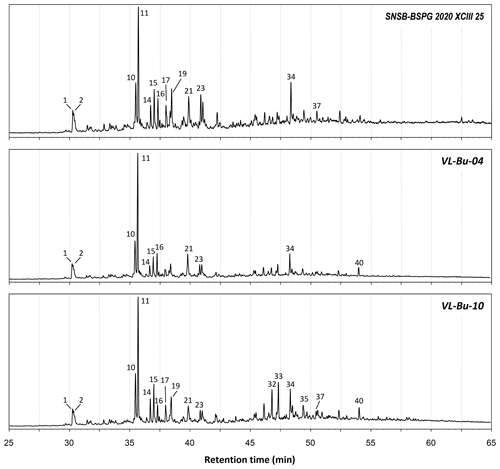
The total ion chromatograms of the thermochemolysis–gas chromatography–mass spectrometry (THM–GC–MS) analysis of the Burmese resins are illustrated in Figs. 1 and B2 (see also Fig. 2 for a comparison of the reconstructed chromatograms of Baltic and Burmese amber). The identified compounds, their fragments used for integration, and their mass spectra factors are listed in Table A1. The relative proportions of the (i) different categories of compounds and (ii) labdanoid diterpenes and compositional ratios are given in Table 2.
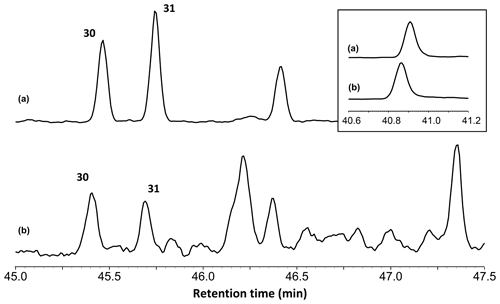
Figure 2Reconstructed chromatogram (m∕z = 173+175) of Baltic amber (a) and the Burmese amber VL-Bu-04 (b). The similar retention times of compounds 30 and 31 in Baltic and Burmese ambers indicate the regular configuration of labdanoid diterpenes. The box depicts the similar retention time for the most intense trimethylnaphthalene isomer (m∕z = 155). The slight differences in retention times for trimethylnaphthalene (box) and compounds 30 and 31 are experimental deviations due to the non-automated injection.
3.2 Systematic paleontology
-
Order Hymenoptera Linnaeus, 1758
-
Family Rhopalosomatidae Ashmead, 1896
-
Rhopalosomidæ Ashmead 1896: 303. Type genus: Rhopalosoma Cresson, 1865. (Ashmead preferred his form of the family name to Rhopalosomatidæ (see footnote on page 303). In doing so, he used an incorrect spelling for the stem of his proposed new family name. Since the correct combining stem is “Rhopalosomat-”, the family name was consequently corrected to Rhopalosomatidae by Brues (1922)).
-
Rhopalosomatidae Ashmead: Brues, 1922: 102 (and most subsequent authors).
Diagnosis (modified from Krogmann et al., 2009)
Among aculeate Hymenoptera, Rhopalosomatidae can be easily identified by the following combination of characters: basal flagellomeres with distinct apical bristle(s) (Figs. 3a, d, 4d, 5b, 6c–e; very small or completely reduced in many Olixon), mesosternum with posterad paired lobes covering the bases of the mesocoxae (Figs. 3c, 4c), male parameres upcurved (Fig. 3e), female sting upcurved (Fig. 3b), female tarsomeres laterally widened (Fig. 5d), female pretarsus with arolium and cuticular plate greatly enlarged and claw sensor elongated.
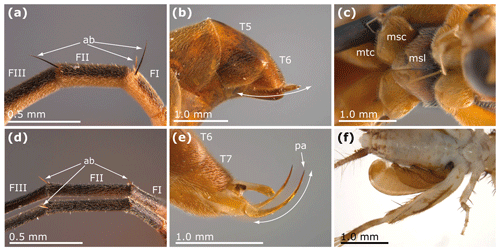
Figure 3Some diagnostic characters of Rhopalosomatidae. (a) Liosphex bribri Lohrmann (in Lohrmann and Ohl, 2010), female, paratype, apical bristles on basal flagellomeres. (b) Paniscomima kilombero Lohrmann, 2011, female, paratype, last metasomal segments with upcurved sting. (c) Liosphex varius Townes, 1977, male, mesosternal lobes covering the bases of the mesocoxae. (d) Olixon ferrugineum Krogmann et al., 2009, female, paratype, apical bristles on basal flagellomeres. (e) Rhopalosoma poeyi Cresson, 1865, male, last metasomal segments with upcurved parameres. (f) Fourth instar larva of a rhopalosomatid (Olixon?) wasp on a nemobiine cricket (Nemobiinae), collected in Florida, USA (deposited in the personal collection of Volker Lohrmann). Abbreviations: ab – apical bristle; FI, FII, FIII – flagellomere 1, flagellomere 2, flagellomere 3; T5, T6, T7 – metasomal tergite 5, metasomal tergite 6, metasomal tergite 7; msl – mesosternal lobe; msc – mesocoxa; mtc – metacoxa; pa – paramere. A cutout of panel (f) was first published in Fossil Record (Lohrmann and Engel, 2017).
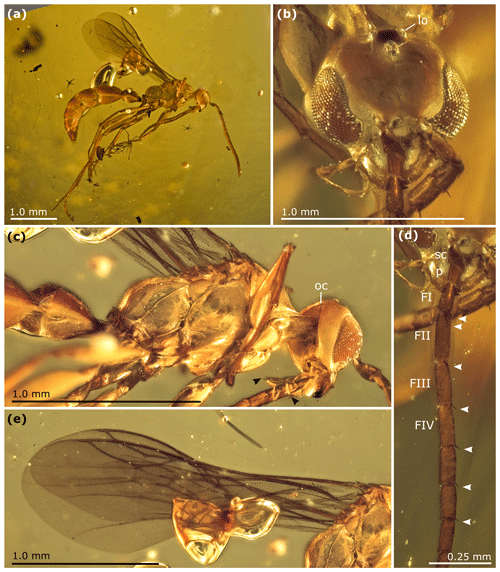
Figure 4Cretolixon alatum gen. et sp. nov., male, holotype (MB.I 6500; ZMB). (a) Habitus in lateral view. (b) Head in slightly oblique frontal view. (c) Mesosoma in lateral view. (d) Partial right antenna. (e) Wings. Abbreviations: ab – apical bristle; FI, FII, FIII – flagellomere 1, flagellomere 2, flagellomere 3; lo – lateral ocellus; oc – occipital carina; p – pedicel; sc – scape. The arrowheads indicate the apical bristles on third maxillary palpomeres (c) and basal flagellomeres (d).
Rhopalosomatid larvae are easily recognized by their habit as ectoparasitoids of true crickets (Gryllidae) (Fig. 3f), unique within Hymenoptera.
With only eight enclosed fore wing cells (C, R, 1Cu, 1R1, 2R1, 1Rs, 1M, and 2Cu), the wing venation of fully winged rhopalosomatid species may superficially resemble those of some other aculeates, e.g., extinct Formiciinae, Sphecomyrminae, and some Myrmeciinae, whereas members of the genus Olixon are brachypterous or apterous. However, Rhopalosomatidae can be distinguished from them by the very narrow fore wing costal cell, the relatively large and oblique fore wing cell 1M, and the position and form of the hind wing veins Rs* (short, straight, and reclivous but angled or curved in most Rhopalosoma) and cu-a (basal or opposite of the branching of veins M and Cu).

Figure 5Cretolixon alatum gen. et sp. nov., female, paratype (VL-Bu-04, UMB). (a) Habitus in lateral view. (b) Head and basal halves of antennae in oblique lateral view. (c) Mesosoma in lateral view. (d) Right protibia and protarsus. (e) Wings. The arrowheads indicate the apical bristles on second labial and third maxillary palpomeres (b).

Figure 6Cretolixon alatum gen. et sp. nov., male and female, paratypes (male: SNSB-BSPG 2020 XCIII 25, SNSB; female: NIGP173865, NIGP). (a, c, d) Male. (a) Habitus in lateral view. (c) Base of right antenna. (d) Base of left antenna. (b, e) Female. (b) Habitus in slightly oblique dorsal view. (e) Partial right antenna (apex of FI to base of FIV). The arrowheads indicate the apical bristles on basal flagellomeres.
Sexual dimorphism
Apart from the genital morphology, males and females can be differentiated by the following: the number of flagellomeres – females have 10, males have 11; the number of visible metasomal tergites – females have 6, males have 7; the form of the tarsomeres II–IV – cylindrical for males and dorsoventrally flattened for females; the absence or presence of tarsal plantulae – absent in females, present in males; and the form of the apex of the pretarsal claws – simple in females, bifid in males.
-
Genus Cretolixon Lohrmann gen. nov.
-
LSID (genus): urn:lsid:zoobank.org:act:47D641BE-A9C1-4ED2-BE34-BA0D20B1766B
-
LSID (author): urn:lsid:zoobank.org:author:05A758C9-462A-422C-B8D6-DD9530E2BD05
-
Type species: Cretolixon alatum Lohrmann sp. nov.
Diagnosis
Among the rhopalosomatid genera, Cretolixon is unique in showing the following character combination: fully developed wings (Figs. 4–7; Olixon spp. are brachypterous or apterous), hind wing vein M and Cu diverging far beyond cu-a (Fig. 7; distad by at least the length of cu-a; in Eorhopalosoma the hind wing vein M and Cu diverge near the cross vein cu-a), occipital carina present (Fig. 4c; absent in Liosphex), maxillary palpomere 3 with a distinct apical bristle (Fig. 8b; absent in Eorhopalosoma, Liosphex, Paniscomima, and Rhopalosoma), labial palpomere 2 with a distinct apical bristle (Fig. 8c; absent in Eorhopalosoma, most Rhopalosoma, and Olixon) each, moderately small ocelli (OOD about 3.0 times LOD; Figs. 4b, 5b, 6b; minute in Olixon, large to very large in Eorhopalosoma, Rhopalosoma, and Paniscomima except for P. seyrigi), and slightly concave inner eye margin (Fig. 4b; deeply emarginated or notched in Eorhopalosoma, Liosphex, Paniscomima, and Rhopalosoma).
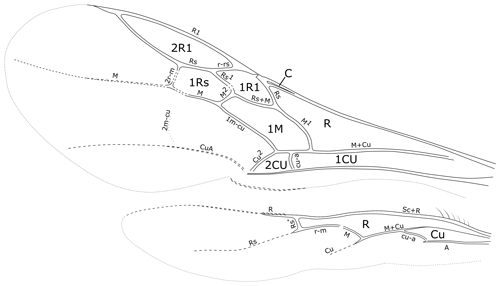
Figure 7Cretolixon alatum gen. et sp. nov., male, holotype (MB.I 6500; ZMB). Venation of fore and hind wings (microtrichia omitted, drawing based on a photograph with a perspective similar to that of Fig. 4e).
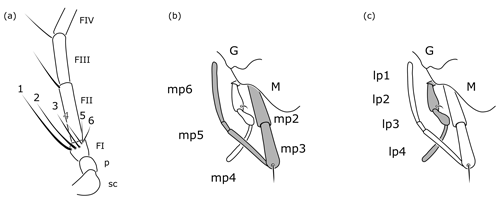
Figure 8Cretolixon alatum gen. et sp. nov., female, paratype (VL-Bu-04; UMB). (a) Basal half of left antenna. (b) Details of mouth parts (maxillary palpus highlighted in grey). (c) Details of mouth parts (labial palpus highlighted in grey). Abbreviations: FI, FII, FIII – flagellomere 1, flagellomere 2, flagellomere 3; G – gena; lp1, …, lp4 – labial palpomere 1, …, labial palpomere 4; M – mandible; mp2, …, mp6 – maxillary palpomere 2, …, maxillary palpomere 6; p – pedicel; sc – scape. Drawings based on Fig. 5b.
Additionally, females of Cretolixon can be easily identified by the six apical bristles on the first flagellomere (Figs. 5b, 6e, 8a) – the maximum number of apical bristles on flagellomere I in any other known rhopalosomatid wasp is two (as in Fig. 3a, d).
Description
Male
Head. Labial palpus four-segmented, basal three palpomeres each distinctly shorter than fourth segment. Labial palpomere 2 with thin apical bristle, less prominent than on maxillary palpomere 3 (as in Fig. 8b, c). Maxillary palpus six-segmented, basal three palpomeres distinctly shorter than apical three palpomeres and slightly broadening towards apex. Maxillary palpomere 3 with distinct apical bristle (as in Fig. 8b, c). Free margin of clypeus slightly concave. Ventral margin of labrum convex, obtusely angled. Mandible quadri/tridentate? (a fourth tooth may be present; however, the perspective makes a validation impossible), its apical teeth infuscate. Scapus clavate. FI conical, FII–X more or less cylindrical, FXI apically pointed. FI with two prominent apical bristles, FII–VI each with a single prominent apical bristle (Fig. 4d). Longer bristle on FI almost as long as flagellomere. FI shorter than FII, which is the longest flagellomere. FIII–X becoming sequentially shorter from base to apex. FXI slightly longer than FX. FI 3.0×, FII 3.3×, and FVII 2.8× as long as wide. Inner eye margin slightly concave at level of antenna (but not deeply emarginate; Fig. 4b). Frons protruding above antennae, with two anterad projections overlapping insertion of antennae. Ocelli moderately small (Fig. 4b). TL 2.0×, OOD 3.0×, MOD 1.0× LOD. Ocellar triangle infuscate (Fig. 4b). Occipital carina present (Fig. 4c). Occiput concave (Fig. 4c).
Mesosoma. Pronotum rather long, anterad strongly narrowing in dorsal view, dorsal posterior margin strongly concave. Notaulus apparently absent. Parapsidal sulcus straight, slightly longer than half of mesoscutal length, reaching posterior mesoscutal margin. Mesosternal lobes present, separated from mesosternum by slight depression (but not constricted as in Paniscomima and Rhopalosoma). Metapleural-propodeal suture carinate (Fig. 4c). Propodeum with median slightly longitudinal tubercle on anterior section. Dorsal face of propodeum in lateral view convexly rounded.
Legs. Fore leg with one tibial spur. Middle and hind legs with two tibial spurs each, inner spur of hind leg modified as calcar. Tarsomeres I–IV each with plantar lobe. Pretarsal claws bifid. Arolium present.
Fore wing. With eight enclosed cells: C, R, 1Cu, 1R1, 2R1, 1Rs, 1M, and 2Cu (Figs. 4e, 7). Costal cell very narrow, over complete distance narrower than bordering veins. Pterostigma narrow. Cell 2R1 (marginal cell) short, ending well before apex of wing. Base of vein M + Cu neither tubular nor present as remnant. Vein cu-a slightly curved, distad of M1 by 1.0× length of cu-a. Vein M1 curved, intersecting vein Cu at an angle of almost 90∘. Vein 1m-cu straight. Faint remnant of vein 2m-cu received by distal section of second submarginal cell (1Rs). Vein Rs1 almost straight, about 1.7× length of M2. Cell 1Rs 2.1× as long as high. Vein CuA distinct, CuP barely present but very short and inconspicuous. Anal lobe without adventitious vein. Wing membrane covered and ventral margin fringed with short, evenly distributed setae.
Hind wing. With two complete cells: R and Cu (Fig. 7). Costal cell only present as membranous area anterior to Sc + R, not enclosed by C. Jugal lobe absent. Anal vein tubular and pigmented, ending at cu-a. Sc + R tubular and pigmented, at basal two-thirds fused with C. Vein R tubular not reaching further than distal hamuli. Vein Rs tubular, straight and slightly reclivous at basal section (Rs*), distal section not tubular but weakly pigmented; pigmentation distally fading. Vein M + Cu tubular and slightly pigmented. Vein Cu not tubular but markedly pigmented, not reaching distal half of wing membrane. Vein M basally tubular but not pigmented, diverging from vein M + Cu by its length beyond cu-a, ending even before connecting with r-m. Vein cu-a oblique, tubular but not pigmented. Five to six basal hamuli straight, their length continuously increasing distad. Five to six distal hamuli. Distal hamuli curved, hook-shaped; similar in size and form. Wing membrane covered and ventral margin fringed with short, evenly distributed setae.
Metasoma. Segment I less than 1.5× as long as wide. Posterior half of tergite I with median depression broadening posterad. Cercus paddle-shaped and with apical setae. Parameres spine-like, upcurved.
Pilosity. Body completely covered with fine pubescence. Pubescence on metasoma slightly longer than on rest of body.
Female
Head, mesosoma, and metasoma as in male except the following: maxillary palpomeres 2 and 3 each with apical stiff bristle. FI very short, about same length as pedicel and one-fifth the length of FII. FI 1.1×, FII 3.9×, and FVII 2.9× as long as wide. FI with six prominent apical bristles along inner margin (Figs. 5b, 6e, 8a), longest bristle (on dorsal side) almost as long as FII, shortest bristle (on ventral side) only as long as FI. FII and FIII with a single prominent apical bristle. TL 2.0×, OOD 2.5×, MOD 1.1× LOD. Fore wing vein Rs1 about 1.6× length of M2. Hind wing with six basal and six distal hamuli. All legs with tarsomere I cylindrical, tarsomeres II–IV dorsoventrally flattened, laterally widened, and distally slightly broadening. Distal margin of tarsomere IV bilobed (Fig. 5d). Tarsomeres ventrally with dense short erect setae. Tarsomeres without plantar lobes and tarsal fenestrae. Tarsomeres I–III with apicolateral bristles. Pretarsal claws simple, without preapical teeth. Arolium large. Sting upcurved.
Etymology
The new genus group name is a combination of “cret-” (referring to Cretaceous) and the name of the recent genus Olixon. The name is neuter.
-
Cretolixon alatum Lohrmann sp. nov.
-
LSID (species): urn:lsid:zoobank.org:act:D47E97C1-EC99-4872-A4BD-314B5496CF2B
-
(registered on 5 December 2019)
-
(Figs. 4–8)
Material
Holotype. Male (Fig. 4), MB.I 6500, deposited in the amber collection of the Museum für Naturkunde Berlin, Berlin, Germany (ex coll. Jens-Wilhelm Janzen), mid-Cretaceous (Albian–Cenomanian) amber from northern Myanmar, Kachin State, Hukawng Valley, Noije Bum Hill, SW of Tanai. The specimen is completely preserved without any significant distortions. The following syninclusions have been observed: one Collembola, Capnodiales, and stellate hairs.
Paratypes. Female (Fig. 5), VL-Bu-04, deposited in the Übersee-Museum Bremen, Bremen, Germany (ex coll. Volker Lohrmann), mid-Cretaceous (Albian–Cenomanian) amber from northern Myanmar, Kachin State, Hukawng Valley. The specimen is almost completely preserved without any significant distortions. The left side of the mesosoma and base of the wings is hidden under an air bubble. Tarsomeres II–V of the right hind leg are missing. In order to access crucial characters of the fossil the amber piece has been cut into two fragments. The following syninclusions have been observed: one Coleoptera (Staphylinidae), one Cicada (nymph), one Blattodea (nymph), plant fibers, and stellate hairs.
Female (Fig. 6b, e), NIGP173865, deposited in the amber collection of the Nanjing Institute of Geology and Palaeontology, Nanjing, China, mid-Cretaceous (Albian–Cenomanian) amber from northern Myanmar, Kachin State, Hukawng Valley. The specimen is incompletely preserved but without any significant distortions. The posterior half of the second metasomal segment and remainder of the metasoma, as well as apical sections of fore wings and hind wings, are missing. Additionally, a fracture is hiding large parts of the anterodorsal mesosoma.
Male (Fig. 6a, c, d), SNSB-BSPG 2020 XCIII 25, deposited in the Staatliche Naturwissenschaftliche Sammlungen Bayerns, Munich, Germany (ex coll. Patrick Müller, BUB 3227), mid-Cretaceous (Albian–Cenomanian) amber from northern Myanmar, Kachin State, Hukawng Valley. The specimen is almost completely preserved without any significant distortions. The following parts of the legs are missing: tarsomeres II–V of right fore leg, apical half of tarsomere II and tarsomeres III–V of left middle and right hind legs, and tarsus and tibial spurs of left hind leg. Additionally, several air bubbles hide parts of the left lower mesosoma and parts of the wing. The following syninclusions have been observed: parts of a filiform antenna and leg of an unidentified insect, stellate hairs, and plant fibers.
Diagnosis
As for the genus (vide supra).
Description
As for the genus, with the following additions (n.m. = not measurable, due to preservation).
Male (given for the holotype (MB.I 6500) first followed by the measurements of the paratype (SNSB-BSPG 2020 XCIII 25) in parentheses). Total body length (head plus mesosoma plus metasoma) in lateral view 3.69 mm (4.00 mm; head measured at slightly oblique angle). Head width (in dorsal view) 0.54 mm (0.63 mm; in ventral view). Antennal length 1.79 mm (1.83 mm) (flagellar length 1.56 mm (1.58 mm)). Mesosomal length in lateral view 1.16 mm (1.23 mm). Fore wing length 2.36 mm (2.42 mm). Hind wing length 1.79 mm (1.75 mm). Fore leg lengths: coxa 0.24 mm (n.m.); trochanter 0.15 mm (n.m.); femur 0.55 mm (0.55 mm); tibia 0.35 mm (n.m.); tarsus 0.78 mm (n.m.). Middle leg lengths: coxa 0.24 mm (0.25 mm); trochanter 0.15 mm (0.175 mm); femur 0.58 mm (0.63 mm); tibia 0.53 mm (0.58); tarsus 0.97 mm (0.85 mm; measured at oblique angle). Hind leg lengths: coxa 0.36 mm (n.m.); trochanter 0.15 mm (0.15 mm); femur: 0.72 mm (0.75 mm); tibia 0.78 mm (0.78 mm); tarsus 1.36 mm (n.m.). Metasomal length in lateral view (slightly expanded in holotype) 2.28 mm (2.40 mm).
Female (due to preservation only given for VL-Bu-04). Total body length (head plus mesosoma plus metasoma) in lateral view 5.16 mm. Head width in dorsal view 0.68 mm. Antennal length 2.2 mm (flagellar length 1.9 mm). Mesosomal length in lateral view 1.48 mm. Fore wing length 2.6 mm. Hind wing length 2.00 mm. Fore leg lengths: coxa 0.3 mm, trochanter 0.15 mm, femur 0.55 mm, tibia 0.56 mm, tarsus 1.13 mm. Middle leg lengths: coxa 0.25 mm, trochanter 0.13 mm, femur 0.8 mm, tibia 0.73 mm, tarsus 1.3 mm. Hind leg lengths: coxa 0.38 mm, trochanter 0.23 mm, femur 0.95 mm, tibia 0.93 mm, tarsus 1.68 mm. Metasomal length in lateral view 3.15 mm.
Etymology
The specific epithet alatum (ala (L.) = wing) refers to the presence of fully developed wings.
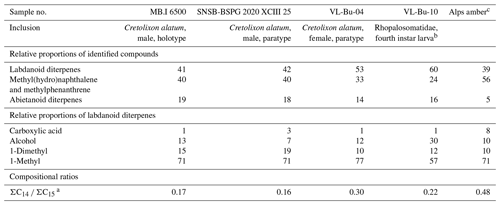
Chemical analysis and the origin and botanical source of the amber
Our thermochemolysis of the investigated fossils revealed chromatograms that are dominated by bicyclic products. These products represented 41 % (MB.I 6500) to 60 % (VL-Bu-10) of the identified compounds, which is characteristic of class I ambers. The retention times of the methyl esters of C15 labdanoids were similar in Burmese ambers and in the reference Baltic amber, which indicated that these acids were derived from polymerized communic acid and a regular configuration of labdanoid diterpenes, typical of class Ia and Ib ambers (Fig. 2). The absence of succinic acid classified Burmese ambers as class Ib resins (Anderson, 1994). Among labdanoid diterpenes, the main products were 1-methylbicyclic hydrocarbons that are supposed to derive from A-ring defunctionalization of the polylabdanoid structure (Anderson, 1995). These compounds represented 57 % (VL-Bu-04) to 77 % (SNSB-BSPG 2020 XCIII 25) of labdanoid diterpenes. Their proportion is assumed to increase with maturation (Clifford et al., 1999). Compared with other Cretaceous and Triassic class Ib ambers, the large predominance of 1-methylbicyclic hydrocarbons among labdanoid diterpenes appears as a characteristic element of the Burmese class Ib ambers since it has already been described for a Cretaceous amber from Myanmar (= mid-Cretaceous Kachin amber; Dutta et al., 2011). The chemical composition of these ambers clearly differs (i) from Raritan (Anderson, 2006), Austrian (Triassic; Fischer et al., 2017), and Canadian (Poulin and Helwig, 2015) ambers whose pyrograms are dominated by the methyl ester of C15 labdanoids and (ii) from the probable pinaceous resin from the Isle of Wight whose pyrogram is dominated by abietane diterpenes and whose main compound is dehydroabietic acid methyl ester (Bray and Anderson, 2008). However, it cannot be used as a diagnostic chemical composition since a Cretaceous French amber from the Alps mountains also exhibits this predominance (Nohra et al., 2015; see also Table 2).
Compositional ratios calculated on labdanoid diterpenes distributions and based on the relative proportions of C14 over C15 bicyclic products ranged from 0.2 (SNSB-BSPG 2020 XCIII 25) to 0.3 (VL-Bu-04), which highlights the predominance of C15 compounds. This type of ratio has been suggested to decrease with maturation (Anderson et al., 1992; Clifford et al., 1999). Such values for these compositional ratios classified these ambers in the lower range of previously described Tertiary class I resinites (Clifford et al., 1999).
The second most abundant category of compounds produced by the thermochemolysis of Burmese ambers comprise methyl(hydro)naphthalene and methylphenanthrene. This category of compounds included sesquiterpenoids 5,6,7,8-tetrahydrocadalene (22) and norcadalene (29) and diagenetic products of sesquiterpenoids and diterpenoids. These molecules represented 24 % (VL-Bu-10) to 40 % (MB.I 6500 and SNSB-BSPG 2020 XCIII 25) of the identified compounds, and their proportion is assumed to increase with maturation (Clifford and Hatcher, 1995). Together with the high proportions of 1-methylbicyclic hydrocarbons and the low compositional ratios for labdanoid diterpenes, this relatively high proportion of methyl(hydro)naphthalene and methylphenanthrene could indicate that Burmese ambers are characterized by a high degree of maturation.
The compounds of the third category are abietane diterpenes including 16,17,19-trisnorabieta-8,11,13-triene (34); 16,17,18-trisnorabieta-8,11,13-triene (35); 18,19-bisnorsimonellite (36) and one of its isomers (39); 16,17-bisnordehydroabietane (37); 7-oxo-16,17,18-trisnorabieta-8,11,13-triene (38); 16,17-bisnordehydroabietic acid methyl ester (44); and 8-abieten-18-oic acid methyl ester (47) which was only detected in VL-Bu-04. Abietane diterpenes represented 14 % (VL-Bu-04) to 19 % (MB.I 6500) of the identified compounds. Three of the four most intense abietanes (34, 36, and 37) were also described in the late Cretaceous (Campanian) Tilin amber from central Myanmar (Zheng et al., 2018).
In conclusion, the results of THM–GC–MS highlight the amber rather than copal nature of these materials. Their chemical compositions correspond to class Ib ambers that would have undergone a relatively high degree of maturation. They are in accordance with previously published analyses of Burmese Cretaceous ambers from Kachin (e.g., Dutta et al., 2011) and are clearly distinct from Campanian Tilin amber (Zheng et al., 2018). So far, Hkamti amber has not been analyzed by THM–GC–MS, but it is significantly older than Kachin amber (Xing and Qiu, 2020) and should arguably be distinct as well. The attested origin of the holotype MB.I 6500, from Noije Bum Hill, supports the same origin as this or another site of the Hukawng Valley for the three other specimens.
Cretolixon and Rhopalosomatidae
Cretolixon is an enigmatic new genus, which can be confidently assigned to the crown group rhopalosomatids. Specifically, the presence of diagnostic characters such as a very narrow fore wing costal cell, distinct apical bristles on the basal flagellomeres, posterad paired mesosternal lobes covering the bases of the mesocoxae, laterally widened female tarsomeres, upcurved parameres, and an upcurved sting support the placement of the genus in the family. On the other hand, a placement among the two recent subfamilies is far more challenging and without a phylogenetic analysis rather difficult. Thus, we choose a conservative approach and will test the placement of Cretolixon in a future study. However, in the following, we will discuss key characters and their implications for a potential subfamily affinity in more detail (see also Figs. 9 and 10).
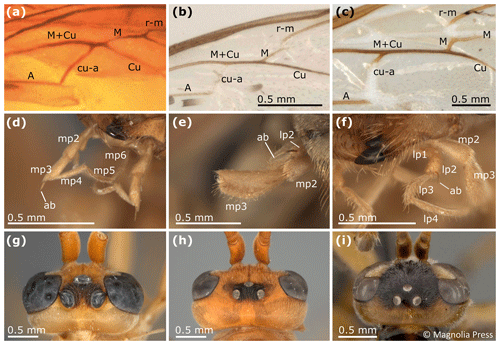
Figure 9Some characters of Rhopalosomatidae. (a) Rhopalosomatidae, gen. et sp. indet., Burmese (Kachin) amber (ZMB; MB.I 6501), basal section of hind wing. (b) Liosphex boreus Lohrmann (in Lohrmann and Ohl, 2010), basal section of hind wing. (c) Rhopalosoma nearcticum Brues, 1943, basal section of hind wing. (d) Olixon banksii (Brues, 1922), mouthparts. (e) Liosphex longicornis Lohrmann (in Lohrmann and Ohl, 2010), mouthparts. (f) Paniscomima kilombero Lohrmann, 2011, mouthparts. (g) Paniscomima opposita Guidotti, 2007, male, paratype, head in dorsal view. (h) Paniscomima seyrigi (Berland, 1951), female, head in dorsal view. (i) Liosphex varius Townes, 1977, female, holotype, head in dorsal view. Abbreviations: ab – apical bristle; lp1, …, lp4 – labial palpomere 1, …, labial palpomere 4; mp1, …, mp6 – maxillary palpomere 1, …, maxillary palpomere 6. Panel (i) is reproduced with permission from copyright holder and was first published in Zootaxa (Lohrmann and Ohl, 2010); photo by David Wahl.
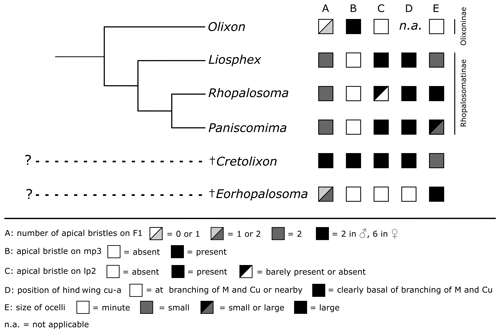
Figure 10Distribution of the discussed characters among Rhopalosomatidae. The tree topology of the recent genera is based on Brothers and Carpenter (1993), Brothers (1999) (both: “rhopalosomatids” sister to Olixon), Guidotti (1999), and unpublished molecular data (Blaschke et al., unpublished results) (both: Olixon + (Liosphex + (Rhopalosoma + Paniscomima))).
Cretolixon – a common ancestor of Rhopalosomatinae and Olixoninae?
As it would “undoubtedly” render the Rhopalosomatinae paraphyletic, Krogmann et al. (2009) argued against Engel's subfamily classification, thus ignoring earlier morphological studies supporting a sister group relationship between the macropterous rhopalosomatids and the brachypterous Olixon, i.e., Brothers and Carpenter (1993), Brothers (1999), and Guidotti (1999). Brothers and Carpenter (1993) and Brothers (1999), based on their phylogenetic analyses, list nine potential synapomorphies for the Rhopalosomatinae. However, since most of these characters are not applicable to the morphologically aberrant Olixon it remains unclear whether these character states are novelties for the subfamily or whether they have evolved in the stem of the family with a secondary loss or derivation in the Olixoninae. The same might be true for the 22 characters received by Guidotti (1999) in her analysis of the generic relationships of rhopalosomatid wasps.
One of Guidotti's potential apomorphies for the Rhopalosomatinae is a stiff apical bristle on the second labial palpomere (Fig. 9e, f). Olixon has a similar structure on the third maxillary palpomere (Fig. 9d) that has also been interpreted as a novelty for the genus by Guidotti (1999). The discovery of both apical bristles, i.e., one each on the second labial and the third maxillary palpomere (Fig. 8b, c), in Cretolixon alatum, leads to the conclusion that both bristles were already present in the common ancestor of both groups. Thus, it is the reduction of one or the other of these bristles that adds to the morphological evidence for the monophyly of each subfamily, respectively. The innovation itself likely occurred earlier in the common ancestor (Fig. 10).
Krogmann et al. (2009) postulated that the single long bristle on the apex of the first flagellomeres of some Olixon species (Fig. 3d) is homologous to one of those two apical bristles present in the recent macropterous genera (Fig. 3a). For the first flagellomere, however, this line of arguments needs a slight modification. The single long bristle on the apex of flagellomere I of some Olixon species is most likely homologous to one of those two apical bristles present in the macropterous genera, and these again are most likely homologous to two of the six found in female Cretolixon (Figs. 5b, 6e, 8a).
Another important character of Cretolixon is the position of the hind wing cross vein cu-a (Fig. 7), which is, similarly to its position in recent genera, clearly basal to the branching of the veins M and Cu (similar to Fig. 9b, c). In Eorhopalosoma and most of the unpublished Burmese amber fossils of the family the cross vein cu-a is opposite or very near to the branching of the veins M and Cu (similar to Fig. 9a). Brothers (1975) and Brothers and Carpenter (1993) regarded the basal position of the cross vein cu-a as the ancestral character state among aculeates and a distal position as the derived state but mentioned that several reversals back to the ancestral state occurred throughout the aculeates, and this might be true for Cretolixon and the recent rhopalosomatid genera as well.
Cretolixon and its implication for the biology of early rhopalosomatids
Lohrmann and Engel (2017) showed that the ectoparasitoid larval habit of rhopalosomatids has not changed since the Cretaceous, and the discovery of enlarged ocelli in Eorhopalosoma (Engel, 2008; Boudinot and Dungey, 2020) indicates that early members of the family may have had crepuscular or nocturnal habits similar to those of recent rhopalosomatids. In contrast to Eorhopalosoma and most species of Paniscomima and Rhopalosoma (similar to Fig. 9g), Cretolixon has moderately small ocelli (Figs. 4b, 5b, 6b); i.e., the size is similar to those found in Liosphex (Fig. 9i). Otherwise, only Paniscomima seyrigi (Berland, 1951) has ocelli of a similar size (Fig. 9h). While this is surely the plesiomorphic character state within the family, it does not allow any conclusion with respect to the biology of the species.
The two previously described Burmese rhopalosomatid taxa are known from males only. Thus, C. alatum gen. et sp. nov. represents the first Cretaceous rhopalosomatid species known from both sexes and thus allows for investigations of sexual dimorphism. As known from recent Rhopalosomatidae, Cretolixon shows only moderate sexual dimorphism. Apart from the reproductive organs, the visible differences are limited to the number of visible metasomal tergites and the morphology of the antennae and legs.
With its unique mixture of characters, Cretolixon gen. nov. represents a significant piece of evidence for our understanding of the early evolution of Rhopalosomatidae. Cretolixon not only provides further evidence for the monophyly of the family but also contributes evidence for the monophyly of the Rhopalosomatinae. A robust phylogenetic placement of the new genus, however, is not possible without a detailed phylogenetic study, which will be part of an ongoing collaboration including morphological and molecular data.
Taxonomic and ethical statement
The current contribution includes revised parts of a chapter used earlier in the PhD thesis of Volker Lohrmann, i.e., the description of the male holotype of Cretolixon alatum as well as Fig. 3. Because the thesis includes a statement disclaiming all relevant nomenclatural acts for taxonomic use, the new taxon is formally described here to make its name available according to the ICZN (1999).
We are well aware of the ethical problems linked to the study of Burmese amber that originates from excavation sites in Kachin State, Myanmar (e.g., Sokol, 2019). We agree with the general intentions of two recently published opinions, i.e., Haug et al. (2020) and Szwedo et al. (2020), and declare that all Kachin amber pieces included in this study are deposited in public collections and have been collected and subsequently traded before November 2017 – the time when the military troubles in the amber mine area of Kachin State started (Human Rights Council, 2019).
Figure B1Molecules identified in the pyrolysates of Burmese amber. Individual compounds are labeled according to the identification of peaks in Figs. 1 and B2 and Table A1.
The four type specimens of Cretolixon alatum are deposited in the Museum für Naturkunde (Berlin, Germany), the Nanjing Institute of Geology and Palaeontology, CAS (Nanjing, China), the Bayerische Staatssammlung für Paläontologie und Geologie, SNSB (München, Germany), and the Übersee-Museum Bremen (Bremen, Germany).
VL designed the study. QZ, PMü, and VL provided fossils. VL and VP carried out the preparation of the fossil amber samples for the THM–GC–MS analysis and for microscopic studies of the fossils. LJ did the chemical analysis. VL prepared the taxonomic descriptions. JB provided unpublished molecular data integrated in Fig. 10. PMi prepared the photographs of the fossils. VL, VP, and LJ wrote the first draft of the paper. All authors contributed to the discussion and proofreading of the manuscript.
The authors declare that they have no conflict of interest.
We sincerely thank the late Jens-Wilhelm Janzen (Seevetal, Germany) and Barbara Odifredi (Carrara, Italy) for making two of the herein newly reported amber fossils available and David Grimaldi (American Museum of Natural History, New York, USA), Bo Wang (Nanjing Institute of Geology and Palaeontology, Nanjing, China), and Christian Neumann (Museum für Naturkunde, Berlin, Germany) for the loan of material. Furthermore, we thank Brendon Boudinot (University of California, Davis, USA) for double-checking important characters of the type of Eorhopalosoma lohrmanni, Takuma Yoshida (Hokkaido University, Sapporo, Japan) and his team for sharing their knowledge of the first record of Paniscomima from the Palearctic Region, and Sieghard Ellenberger (Kassel, Germany) for information on the holotype's origin. For their generous financial support enabling the UMB to purchase additional rhopalosomatid inclusions in Burmese amber, we would like to thank the Petra und Joachim Schaffer-Stiftung (Bremen, Germany) and Sabine Stuth (Bremen, Germany), as well as Irmgard and Nils Kadelbach (Bremen, Germany). Finally, we thank the reviewers, Denis J. Brothers (University of KwaZulu-Natal, Scottsville, South Africa) and Alexandr P. Rasnitsyn (Russian Academy of Sciences, Moscow, Russia), and the editor, Alexander Schmidt (University of Göttingen, Germany), for their valuable comments and time spent on the manuscript.
Financial support in the initial and final stage of this project was provided by the Studienstiftung des Deutschen Volkes (to Volker Lohrmann), grant OSUR from program AO2 2018 for visiting researchers (to Vincent Perrichot, enabling Volker Lohrmann's work at the University of Rennes 1) and by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000) and the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0706; both to Bo Wang and enabling the purchase of the NIGP specimen studied herein and Vincent Perrichot's work at NIGP, respectively).
This paper was edited by Alexander Schmidt and reviewed by Denis J. Brothers and Alexandr P. Rasnitsyn.
Aguiar, A. P., Deans, A. R., Engel, M. S., Forshage, M., Huber, J. T., Jennings, J. T., Johnson, N. F., Lelej, A. S., Longino, J. T., Lohrmann, V., Mikó, I., Ohl, M., Rasmussen, C., Taeger, A., and Yu, D. S. K.: Order Hymenoptera, Zootaxa, 3703, 51–62, https://doi.org/10.11646/zootaxa.3703.1.12, 2013.
Anderson, K. B.: The nature and fate of natural resins in the geosphere – IV. Middle and Upper Cretaceous amber from the Taimyr Peninsula, Siberia – evidence for a new form of polylabdanoid of resinite and revision of the classification of Class I resinites, Org. Geochem., 21, 209–212, https://doi.org/10.1016/0146-6380(94)90155-4, 1994.
Anderson, K. B.: New evidence concerning the structure, composition, and maturation of class I (Polylabdanoid) resinites, in: Amber, resinite, and fossil resins, edited by: Anderson, K. B. and Crelling, J. C., Am. Chem. Soc. Symposium Series, 617, 105–129, 1995.
Anderson, K. B.: The nature and fate of natural resins in the geosphere – XII. Investigation of C-ring aromatic diterpenoids in Raritan amber by pyrolysis-GC-matrix isolation FTIR-MS, Geochem. Trans., 7, 2, https://doi.org/10.1186/1467-4866-7-2, 2006.
Anderson, K. B., Winans, R. E., and Botto, R. E.: The nature and fate of natural resins in the geosphere – II. Identification, classification and nomenclature of resinites, Org. Geochem., 18, 829–841, https://doi.org/10.1016/0146-6380(92)90051-X, 1992.
Archibald, S. B., Rasnitsyn, A. P., Brothers, D. J., and Mathewes, R. W.: Modernisation of the Hymenoptera: ants, bees, wasps, and sawflies of the early Eocene Okanagan Highlands of western North America, Can. Entomol., 150, 205–257, https://doi.org/10.4039/tce.2017.59, 2018.
Ashmead, W. H.: Rhopalosomidae [sic!], a new family of fossorial wasps, P. Entomol. Soc. Wash., 3, 303–310, 1896.
Berland, L.: Note sur l'existence à Madagascar de Rhopalosomidae [sic!] (Hymenoptères Aculéates), Mém. L'Inst. Scien. Madagascar, Série A, 5, 295–303, 1951.
Boudinot, B. E. and Dungey, D. R.: †Eorhopalosoma lohrmanni, a new species of Rhopalosomatidae from mid-Cretaceous amber of northern Myanmar (Hymenoptera, Aculeata: Vespoidea), Cretaceous Res., 108, 104339, https://doi.org/10.1016/j.cretres.2019.104339, 2020.
Brady, S. G., Larkin, L., and Danforth, B. N.: Bees, ants and stinging wasps (Aculeata), in: The Timetree of Life, edited by: Hedges, S. B. and Kumar, S., Oxford Univ. Press, 264–269, 2009.
Branstetter, M. G., Danforth, B. N., Pitts, J. P., Faircloth, B. C., Ward, P. S., Buffington, M. L., Gates, M. W., Kula, R. R., and Brady, S. G.: Phylogenomic analysis of ants, bees and stinging wasps: improved taxon sampling enhances understanding of Hymenopteran evolution, Curr. Biol., 27, 1019–1025, https://doi.org/10.1016/j.cub.2017.03.027, 2017.
Bray, P. S. and Anderson, K. B.: The nature and fate of natural resins in the geosphere XIII: a probable pinaceous resin from the early Cretaceous (Barremian), Isle of Wight, Geochem. Trans., 9, 3, https://doi.org/10.1186/1467-4866-9-3, 2008.
Brothers, D. J.: Phylogeny and classification of the aculeate Hymenoptera, with special reference to Mutillidae, Univ. Kans. Sci. Bull., 50, 483–648, 1975.
Brothers, D. J.: Phylogeny and evolution of wasps, ants and bees (Hymenoptera, Chrysoidea, Vespoidea and Apoidea), Zool. Scr., 28, 233–249, 1999.
Brothers, D. J. and Carpenter, J. M.: Phylogeny of Aculeata: Chrysidoidea and Vespoidea, J. Hym. Res., 2, 227–304, 1993.
Brown, F. M.: Lithoserix williamsi (Siricidae, Hymenoptera), a newly recognized fossil horntail from Florissant, Colorado, Insecta Mundi, 1, 119–120, 1986.
Brues, C. T.: On the hymenopterous genus Harpagocryptus and its allies, Psyche, 29, 101–109, 1922.
Brues, C. T.: The American species of Rhopalosoma, Ann. Entomol. Soc. Am., 36, 310–318, 1943.
Cameron, P.: Fam. Braconidae, Olixon, in: Biologia Centrali-Americana, Insecta, Hymenoptera (Families Tenthredinidae – Chrysididae), Vol. 1, by: Cameron, P., 412–413 + plate 16, 1887.
Clifford, D. J. and Hatcher, P. G.: Structural transformations of polylabdanoid resinites during maturation, Org. Geochem., 23, 407–418, https://doi.org/10.1016/0146-6380(95)00022-7, 1995.
Clifford, D. J., Hatcher, P. G., Botto, R. E., Muntean, J. V., and Anderson, K. B.: The nature and fate of natural resins in the geosphere. IX1For the last entry in this series see Clifford et al. (1998), 1: Structure and maturation similarities of soluble and insoluble polylabdanoids isolated from Tertiary Class I resinites, Org. Geochem., 30, 635–650, https://doi.org/10.1016/S0146-6380(99)00018-2, 1999.
Cockerell, T. D. A.: Fossils in Burmese amber, Nature, 109, 713–714, 1922.
Cresson, E.: On the Hymenoptera of Cuba, Proc. Entomol. Soc. Phila., 4, 1–200, 1865.
Darling, D. C. and Sharkey, M. J.: Order Hymenoptera, in: Insects from the Santana Formation, Lower Cretaceous, of Brazil, edited by: Grimaldi, D. A., Bull. Am. Mus. Nat. Hist., 195, 209–229, 1990.
Dlussky, G. M. and Rasnitsyn, A. P.: Two new aculeate hymenopterans (Vespida = Hymenoptera) from the Middle Eocene of United States, Paleontol. J., 33, 546–549, 1999 (Translated from Paleontol. Zh., 5, 72–75, 1999).
Dutta, S., Mallick, M., Kumar, K., Mann, U., and Greenwood, P. F.: Terpenoid composition and botanical affinity of Cretaceous resins from India and Myanmar, Int. J. Coal Geol., 85, 49–55, https://doi.org/10.1016/j.coal.2010.09.006, 2011.
Enderlein, G.: Paniscomima, eine neue von Herrn Baron von Erlanger aufgefundene Rhopalosomiden-Gattung, Zool. Anz., 27, 464–466, 1904.
Engel, M. S.: The wasp family Rhopalosomatidae in mid-Cretaceous amber from Myanmar (Hymenoptera: Vespoidea), J. Kans. Entomol. Soc., 81, 168–174, https://doi.org/10.2317/JKES-712.11.1, 2008.
Fischer, T. C., Sonibare, O. O., Aschauer, B., Kleine-Benne, E., Braun, P., and Meller, B.: Amber from the Alpine Triassic of Lunz (Carnian, Austria): a classic palaeobotanical site, Palaeontology, 60, 743–759, https://doi.org/10.1111/pala.12313, 2017.
Grimaldi, D. and Engel, M. S.: Evolution of the Insects, Cambridge Univ. Press, Cambridge, 2005.
Guidotti, A. E.: Systematics of little known parasitic wasps of the family Rhopalosomatidae (Hymenoptera: Vespoidea), M.Sc. Thesis, University of Toronto, Canada, 1999.
Guidotti, A. E.: A revision of the wasp genus Paniscomima (Hymenoptera: Rhopalosomatidae) and a proposal of phylogenetic relationships among species, Invertebr. Syst., 21, 297–309, https://doi.org/10.1071/IS04027, 2007.
Gurney, A. B.: Notes on the biology and immature stages of a cricket parasite of the genus Rhopalosoma, Proc. U.S. Nat. Mus., 103, 19–34, 1953.
Haug, J. T., Azar, D., Ross, A., Szwedo, J., Wang, Bo, Arillo, A., Baranov, V., Bechteler, J., Beutel, R., Blagoderov, V., Delclòs, X., Dunlop, J., Feldberg, K., Feldmann, R., Foth, C., Fraaije, R. H. B., Gehler, A., Harms, D., Hedenäs, L., Hyžny, M., Jagt, J. W. M., Jagt-Yazykova, E. A., Jarzembowski, E., Kerp, H., Khine, P. K., Kirejtshuk, A. G., Klug, C., Kopylov, D. S., Kotthoff, U., Kriwet, J., McKellar, R. C., Nel, A., Neumann, C., Nützel, A., Peñalver, E., Perrichot, V., Pint, A., Ragazzi, E., Regalado, L., Reich, M., Rikkinen, J., Sadowski, E.-M., Schmidt, A. R., Schneider, H., Schram, F. R., Schweigert, G., Selden, P., Seyfullah, L. J., Solórzano-Kraemer, M. M., Stilwell, J. D., van Bakel, B. W. M., Vega, F. J., Wang, Y., Xing, L., and Haug, C.: Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated April 21, 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: Myanmar amber, Palaeontol. Z., 94, 413–429, https://doi.org/10.1007/s12542-020-00524-9, 2020.
Hood, J. D.: Notes on the life history of Rhopalosoma poeyi Cresson (Note: additional comments on the paper and rhopalosomatids by Rohwer appear on 147–148), Proc. Entomol. Soc. Wash., 15, 145–147, 1913.
Huber, J. T. and Sharkey, M. J.: chap. 3, Structure, in: Hymenoptera of the world: An identification guide to families, edited by: Goulet, H. and Huber, J. T., Agriculture Canada, Research branch, Publication 1894/E, 13–59, 1993.
Human Rights Council: The economic interests of the Myanmar military, Report A/HRC/42/CRP.3, 1–110, 2019.
ICZN (International Commission on Zoological Nomenclature): International code of zoological nomenclature, 4th Edn., The International Trust for Zoological Nomenclature, London, i–xxix, 306 pp., 1999.
Kasparyan, D. R. and Rasnitsyn, A. P.: On the taxonomic position of Lithoserix williamsi Brown (Hymenoptera: Ichneumonidae) from the Oligocene of Colorado (USA), Paleontol. J., 26, 105–106, 1992.
Krogmann, L., Austin, A. D., and Naumann, I. D.: Systematics and biogeography of Australian rhopalosomatid wasps (Hymenoptera: Rhopalosomatidae) with a global synopsis of Olixon Cameron, Syst. Ent., 34, 222–251, https://doi.org/10.1111/j.1365-3113.2008.00460.x, 2009.
Linnaeus, C.: Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, Tomus I, Editio decima, reformata, Salvius, Holmiæ, 824 pp., 1758.
Lohrmann, V.: A revision of the Paniscomima of the African subregion with the description of two new species from Malawi and Tanzania (Hymenoptera: Rhopalosomatidae), Zoosyst. Evol., 87, 371–378, https://doi.org/10.1002/zoos.201100014, 2011.
Lohrmann, V. and Engel, M. S.: The wasp larva's last supper: 100 million years of evolutionary stasis in the larval development of rhopalosomatid wasps (Hymenoptera: Rhopalosomatidae), Foss. Rec., 20, 239–244, https://doi.org/10.5194/fr-20-239-2017, 2017.
Lohrmann, V. and Ohl, M.: World revision of the wasp genus Liosphex Townes, 1977 (Hymenoptera: Rhopalosomatidae), Zootaxa, 2384, 1–43, https://doi.org/10.11646/zootaxa.2384.1.1, 2010.
Lohrmann, V., Fox, M., Solis, M., and Krogmann, L.: Systematic revision of the New World Olixon Cameron with descriptions of O. melinsula sp. n. and the hitherto unknown female of O. bicolor (Hymenoptera: Rhopalosomatidae), Deut. Entomol. Z., 59, 259–275, 2012.
Lohrmann, V., Falin, Z. H., Bennett, D. J., and Engel, M. S.: Recent findings of Olixon banksii in the Nearctic with notes on its biology (Hymenoptera: Rhopalosomatidae), J. Kans. Entomol. Soc., 87, 258–260, https://doi.org/10.2317/JKES130820.1, 2014.
Lohrmann, V., Ohl, M., Michalik, P., Pitts, J. P., Jeanneau, L., and Perrichot, V.: Notes on rhopalosomatid wasps of Dominican and Mexican amber (Hymenoptera: Rhopalosomatidae) with a description of the first fossil species of Rhopalosoma Cresson, 1865, Foss. Rec., 22, 31–44, https://doi.org/10.5194/fr-22-31-2019, 2019.
Mason, W. R. M.: Standard drawing conventions and definitions for venational and other features of wings of Hymenoptera, P. Entomol. Soc. Wash., 88, 1–7, 1986.
Mason, W. R. M.: Cubitus posterior in Hymenoptera, P. Entomol. Soc. Wash., 92, 93–97, 1990.
Miller, L. A., Benefield, T. D., Lounsbury, S. A., Lohrmann, V., and Blaschke, J. D.: DNA barcoding of rhopalosomatid larvae reveals a new host record and genetic evidence of a second species of Rhopalosoma Cresson (Hymenoptera: Rhopalosomatidae) in America north of Mexico, J. Hymenopt. Res., 74, 35–46, https://doi.org/10.3897/jhr.74.38276, 2019.
Nel, A.: Descriptions et révisions de trois “Siricidae” fossiles du Cénozoïque (Hymenoptera), Bull. Soc. Entomol. Fr., 96, 247–253, 1991.
Nel, A., Azar, D., and Hervet, S.: A new rhopalosomatid wasps [sic] in the Paleocene of France (Hymenoptera), Ann. Soc. Entomol. Fr., 46, 211–215, https://doi.org/10.1080/00379271.2010.10697660, 2010.
Nohra, Y. A., Perrichot, V., Jeanneau, L., Le Pollès, L., and Azar, D.: Chemical characterization and botanical origin of french ambers, J. Nat. Prod., 78, 1284–1293, https://doi.org/10.1021/acs.jnatprod.5b00093, 2015.
Osten, T.: Hymenoptera: bees, wasps and ants, in: The Crato fossil beds of Brazil, edited by: Martill, D. M., Bechly, G., and Loveridge, R. F., Cambridge University Press, 350–365, 2007.
Perkins, R. C. L.: Some remarkable Australian Hymenoptera, Proc. Hawaiian. Entomol. Soc., 2, 27–35, 1908.
Pilgrim, E. M., von Dohlen, C. D., and Pitts, J. P.: Molecular phylogenetics of Vespoidea indicate paraphyly of the superfamily and novel relationships of its component families and subfamilies, Zool. Scr., 37, 539–560, https://doi.org/10.1111/j.1463-6409.2008.00340.x, 2008.
Poulin, J. and Helwig, K.: Inside amber: New insights into the macromolecular structure of Class Ibresinite, Org. Geochem., 86, 94–106, https://doi.org/10.1016/j.orggeochem.2015.05.009, 2015.
Shi, G., Grimaldi, D. A., Harlow, G. E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., and Li, X.: Age constraint on Burmese amber based on U-Pb dating of zircons, Cretaceous Res., 37, 155–163, https://doi.org/10.1016/j.cretres.2012.03.014, 2012.
Smith, R. D. A. and Ross, A. J.: Amberground pholadid bivalve borings and inclusions in Burmese amber: Implications for proximity of resin producing forests to brackish waters, and the age of the amber, Earth Env. Sci. Trans. R. Soc. Edinburgh, 107, 239–247, https://doi.org/10.1017/S1755691017000287, 2017.
Sokol, J.: Troubled treasure. Fossils in Burmese amber offer an exquisite view of dinosaur times- and an ethical minefield, Science, 364, 722–729, https://doi.org/10.1126/science.aay1187, 2019.
Szwedo, J., Wang, B., Soszyńska-Maj, A., Azar, D., and Ross, A. J.: International Palaeoentomological Society Statement, Palaeoentomology, 3, 221–222, https://doi.org/10.11646/palaeoentomology.3.3.1, 2020.
Townes, H. K.: A revision of the Rhopalosomatidae (Hymenoptera), Contr. Amer. Entomol. Inst., 15, 1–34, 1977.
Wilson, J. S., von Dohlen, C. D., Forister, M. L., and Pitts, J. P.: Family-level divergences in the stinging wasps (Hymenoptera: Aculeata), with correlations to angiosperm diversification, Evol. Biol., 40, 101–107, https://doi.org/10.1007/s11692-012-9189-0, 2013.
Xing, L. and Qiu, L.: Zircon U–Pb age constraints on the mid-Cretaceous Hkamti amber biota in northern Myanmar, Palaeogeogr. Palaeocl., 558, 109960, https://doi.org/10.1016/j.palaeo.2020.109960, 2020.
Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F., Zhang, H., Wang, B., and Dilcher, D.: An ammonite trapped in Burmese amber, P. Natl. Acad. Sci., 116, 11345–11350, https://doi.org/10.1073/pnas.1821292116, 2019.
Zheng, D., Chang, S.-C., Perrichot, V., Dutta, S., Rudra, A., Mu, L., Kelly, R. S., Li, S., Zhang, Q., Zhang, Q., Wong, J., Wang, J., Wang, H., Fang, Y., Zhang, H., and Wang, B.: A Late Cretaceous amber biota from central Myanmar, Nat. Commun., 9, 3170, https://doi.org/10.1038/s41467-018-05650-2, 2018.