the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The first Fulgoridae (Hemiptera: Fulgoromorpha) from the Eocene of the central Qinghai–Tibetan Plateau
Xiao-Ting Xu
Wei-Yu-Dong Deng
Zhe-Kun Zhou
Torsten Wappler
Tao Su
The Qinghai–Tibetan Plateau (QTP) played a crucial role in shaping the biodiversity in Asia during the Cenozoic. However, fossil records attributed to insects are still scarce from the QTP, which limits our understanding on the evolution of biodiversity in this large region. Fulgoridae (lanternfly) is a group of large planthopper in body size, which is found primarily in tropical regions. The majority of the Fulgoridae bear brilliant colors and elongated heads. The fossil records of Fulgoridae span from the Eocene to Miocene in the Northern Hemisphere, and only a few fossil species from Neogene deposits have been reported in Asia so far. Here, we report a new fossil record of Fulgoridae from the middle Eocene Lunpola Basin, central QTP. The specimen is in lateral compression, with complete abdomen, thorax, and part of the wings preserved, while most of the head is missing. It belongs to the “lower Fulgoroidea” judging by several strong lateral spines on the hind tibia and a row of teeth at the apex of the second metatarsomere. This fossil specimen is assigned to Fulgoridae by comparison with nine families of the “lower Fulgoroidea”. The specimen represents the earliest Fulgoridae fossil record in Asia and was considered a new morphotaxon based on the peculiar legs and wings. Based on the modern distribution of fulgorid and other paleontological evidence, we suggest a warm climate with relatively low elevation during the middle Eocene in the central QTP. Therefore, this new fossil record not only provides important information on insect diversity in the middle Eocene, but also gives new evidence on the paleoenvironment in the core area of the QTP from the perspective of an insect.
The Qinghai–Tibetan Plateau (QTP) is the highest and one of the largest plateaus on Earth (Yao et al., 2017). With its unique and complex interactions of atmospheric, cryospheric, hydrological, geological, and environmental processes, the QTP has significantly influenced the Earth's biodiversity, monsoonal climate, and water cycles (Harris, 2006; Yao et al., 2012; Spicer, 2017; Farnsworth et al., 2019). Cenozoic insect fossils have so far been discovered from the QTP, mainly in northeastern Qinghai Province and northern Tibetan regions. A Cixiidae (Fulgoroidea) and a brown lacewing (Hemerobiidae) were reported from the Miocene Garang Formation in northeastern Qinghai Province (Li et al., 2017; Yang et al., 2018). About 20 insect morphotaxa attributed to 18 genera and 12 families were reported from the Paleocene Niubao Formation, Qiangtang Basin (Cai and Fu, 2003; Lin and Huang, 2006; Wang et al., 2019), of which only a few have been formally described, e.g., Flatidae (Szwedo et al., 2013), Lophopidae (Szwedo et al., 2015), and Orthoptera (Lin and Huang, 2006; Wang et al., 2019). These fossil records suggest a warm and humid climate in the Dazhuoma area of Qiangtang Basin in the middle–late Paleocene (Cai and Fu, 2003; Lin and Huang, 2006; Wang et al., 2019) and provide new clues to evolutionary and distributional patterns of these insect groups (Szwedo et al., 2015). In addition, some fossil insects, including Diptera, Hymenoptera, and Heteroptera, have been mentioned in the Quaternary Kongma Basin without detailed description (Shao et al., 2010). Only the water strider, Aquarius lunpolaensis, was formally described from the Niubao Formation in Nima Basin and Lunpola Basin (Lin, 1981; Cai et al., 2019). Generally, the fossil records attributed to insects are still scarce from the QTP, which limits our understanding on the evolution of the biodiversity in this region.
Fulgoromorpha, commonly known as planthopper, covers 21 extant and 12 extinct families (Brysz and Szwedo, 2019), and the oldest confirmed record is from the late Permian of Russia (Martynov, 1935; Szwedo, 2018). The Fulgoromorpha is divided into three superfamilies, among which only Fulgoroidea is extant, and the remaining two are extinct, namely Coleoscytidae and Surijokocixiidae (Szwedo, 2018). Fulgoroidea is the most morphologically variable group among all superfamilies of Auchenorrhyncha, with about 13 665 species in 21 families (Bourgoin, 2021), and its fossil records can be traced back to the Jurassic (Szwedo, 2018). Despite many attempts to create a complete phylogenetic tree of the relation of Fulgoroidea, the systematic relationships among families remain unsolved (Brysz and Szwedo, 2019). Fulgoroidea differs from other Auchenorrhyncha by combining several characters, including frons with distinct longitudinal carinae, tegulae present at the base of the forewings, and forewing anal veins confluent in the basal of claval margin (Dietrich, 2009). This superfamily is a typical group with a feeding behavior of piercing and sucking plant tissue (Denno and Perfect, 1994). Their ethology and ecology are dominated by interactions with their host plants, which served as their food provider, shelter, and ovipositing place (Nault et al., 1985; Denno and Perfect, 1994; Bourgoin et al., 2015).
In the family Fulgoridae, 142 genera and about 774 species are known (Bourgoin, 2021). A total of 11 subfamilies are recognized in the Fulgoridae: Amyclinae, Aphaeninae, Dichopterinae, Enchophorinae, Fulgorinae, Lyncidinae, Phenacinae, Poiocerinae, Strongylodematinae, Xosopharinae, and Zanninae (Bourgoin, 2021). However, as stated by Urban and Cryan (2009), the higher classification of Fulgoridae, which is primarily based on characters of head morphology, was not supported by the molecular phylogeny.
Fulgoridae is mainly distributed in moist tropical regions (Bartlett et al., 2018). The Fulgoridae ranges from 4 mm to 10 cm in length with a wingspan of up to 15 cm (Brambila and Hodges, 2008). Both adults and nymphs often feed on the above-ground parts of dicots (Denno and Perfect, 1994). Many species in this group are brilliantly colored and have an elongated head, reaching more than one-third of the length of the entire body length (Urban and Cryan, 2009).
Fossil records of Fulgoridae are rich and span from the Eocene to the Miocene in the Northern Hemisphere (Wappler, 2003, 2004; Szwedo et al., 2004; Szwedo and Wappler, 2006; Szwedo, 2008). North American and European fossil records are the richest, and many of them are from Eocene deposits (Szwedo et al., 2004). Until now, the Asian records of Fulgoridae fossil have only appeared in the middle Miocene Shanwang Formation in eastern China (Hong, 1979; Zhang, 1989; Zhang et al., 1994).
Here we report a new morphotaxon attributed to the family Fulgoridae from the middle Eocene Lunpola Basin, central QTP. We discuss its systematic assignment, as well as the paleoenvironment of central QTP during the middle Eocene.
The fossil specimen (number XZDY1-I-0031) described herein was collected from the Niubao Formation at Dayu section, Lunpola Basin, Shuanghu County, northern Tibet, China (32.033∘ N, 89.767∘ E; 4655 m a.m.s.l., Fig. 1a).
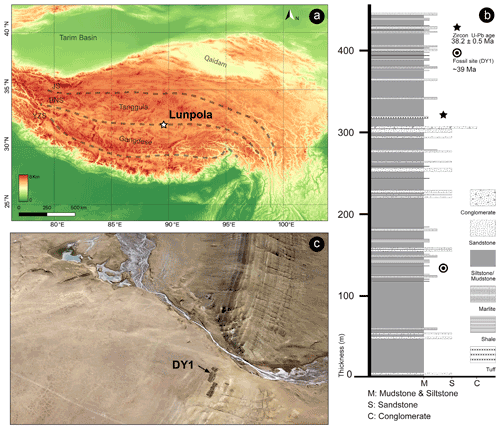
Figure 1Maps showing the location of the specimen. (a) Location of the Lunpola Basin within the Tibetan Plateau terranes. BNS, Bangong–Nujiang suture; JS, Jinshajiang suture; YZS, Yalu–Zangbo suture. Map data provided by SRTM data V4 (Jarvis et al., 2008). (b) Schematic lithologic log of the Dayu section showing stratigraphic positions of radiogenic dating samples and Fulgoridae fossil of the Niubao Formation. Modified from Fang et al. (2020). (c) The specimen was collected from fossil sites DY1 in Lunpola Basin.
The Lunpola Basin is a large Cenozoic sedimentary basin on the central QTP and is situated along the central part of the Bangong–Nujiang suture zone. This basin extends east–west with a length of ∼ 220 km and a width of 15–20 km, initially formed by the Mesozoic collision of the Lhasa and Qiangtang blocks (Sun et al., 2014; Chen et al., 2017). The basin contains sequences of lacustrine to alluvial Cenozoic sediments more than 4000 m thick, which mainly consist of two stratigraphic units: the Niubao Formation exposed in the northern margin of the Lunpola Basin, and the overlying Dingqinghu Formation exposed in the southern margin of the basin (Sun et al., 2012; Wei et al., 2017). The Niubao Formation is about 3000 m thick and mainly consists of three parts: the lower part is composed of brownish red conglomerates, sandstones, and siltstones; the middle part, including our fossil site, is composed of reddish to brownish mudstones, intercalated with thin-bedded fine sandstones, shales, and distinct belted white marlite in basin margins, interpreted mainly as a shallow lake environment; the upper part consists of brownish greyish conglomerates, sandstones, and brownish red mudstones, intercalated with reddish Luvic Paleosols (Fang et al., 2020). The overlying Dingqinghu Formation is about 1000 m thick and is characterized by lacustrine greenish to greyish mudstones, fine siltstones, and oil shales (Sun et al., 2014; Fang et al., 2020).
The age of the site DY1 (Fig. 1c, where our specimen is located) was regarded as ∼ 25.5 Ma in the previous work (e.g., Cai et al., 2019; Su et al., 2019). According to the new chronostratigraphic work using magnetostratigraphic and radiochronologic dating methods, the revised age of the fossil-bearing layer is ∼ 39 Ma (Fig. 1b; Fang et al., 2020).
In the same layer of the fossil site, plenty of plant macrofossils were reported, including Sabalites (Su et al., 2019), Koelreuteria (Jiang et al., 2018), Cedrelospermum (Jia et al., 2018), Limnobiophyllum (Low et al., 2020), Ailanthus (Liu et al., 2019), Pistacia, Handeliodendron, Exbucklandia, Populus, Magnoliales, and Araliaceae (Wu et al., 2017). Besides, vertebrates including climbing perch Eoanabas thibetana (Wu et al., 2017), cyprinid fish Tchunglinius tchangii (Wang and Wu, 2015), and an insect, namely water strider Aquarius lunpolaensis (Lin, 1981; Cai et al., 2019), were reported.
This article is registered in ZooBank (http://www.zoobank.org, last access: 30 July 2021) under LSID LSIDurn:lsid:zoobank.org:pub:2CD563C5-6E6B-4F41-AFE1-162D37780241. The fossil specimen is preserved as a compression. The specimen was observed under a Leica S8AP0 stereomicroscope, and photographs were taken with a Smartzoom 5 digital camera; 70 % alcohol was applied to immerse the fossil to make the morphological details more visible. The nomenclature of wing venation in this research is based on the morphological interpretation and system proposed by Bourgoin et al. (2015). The nomenclature of female terminalia follows Bourgoin (1993). The imprint, specimen XZDY1-I-0031A, and the counterpart, specimen XZDY1-I-0031B, are housed in the Paleoecology Collections, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, China.

Figure 2Fulgoridae gen. et sp. indet. (a) Imprint, specimen XZDY1-I-0031A. (b) Counterpart, specimen XZDY1-I-0031B. CuP, cubitus posterior; cv, crossveins; fg, female genitalia; h, head; hp, horseshoe pattern; hm, hind-wing margins; lpf, left profemur; mf, mesofemurs; ml, metalegs; ms, mesonotum; mt, metanotum; nl, nodal line; p, pronotum; rpf, right profemur; ta, tarsi; ti, tibia.

Figure 3Head, thorax, and legs. (a) Thorax of the part and the counterpart. (b) Preserved part of the head. (c) Prolegs. (d) Metalegs. (e, f) Tarsi of the metalegs. (g) Metatibia of the counterpart. (h) Show the spine in (g). amm, anterior margin of mesonotum; as, apical spines; c, carinae; cl, claw; ls, lateral spines; lt, lateral teeth; mt, median teeth; p, pronotum; pf, profemurs; s, scape; sc, scutellum; te, tegula; ts, tarsomere; v, vertex; w, wrinkles. The specimen was immersed into alcohol except in (a).
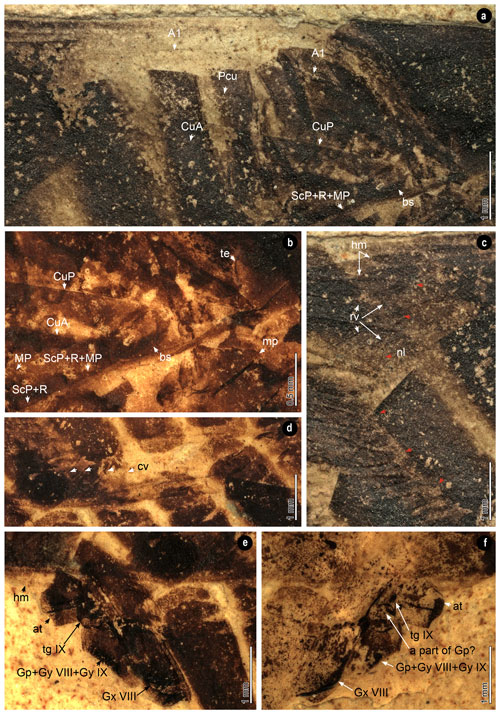
Figure 4Wings and genitalia. (a) Clavus. (b) Base part of the wings. (c) Nodal line and reticular veins of the hind wing. Red arrows trace the nodal line. (d) Crossveins. (e, f) Female genitalia of the part and the counterpart. A1, first anal vein; at, anal tube; bs, basal cell; CuA, cubital anterior; CuP, cubitus posterior; cv, crossveins; Gp, gonoplac; Gx, gonocoxa; Gy, gonapophysis; hm, hind-wing margin; MP, media posterior; mp, metapleuron; nl, nodal line; Pcu, postcubitus; R, radius; rv, reticular veins; ScP, subcosta posterior; te, tegula; tg, tergite. The specimen was immersed into alcohol in (b), (d), (e) and (f).
-
Superfamily Fulgoroidea Latreille
-
Family Fulgoridae Latreille
-
Fulgoridae gen. et sp. indet. (Figs. 2–5)
urn:lsid:zoobank.org:act:03E48F51-B942-4D52-A48A-3DCD66645E6E
Dimensions
The tip of the preserved head to the apex of the forewing 16.4 mm. Pronotum about 1.0 mm long and mesonotum about 1.9 mm long. Forewing about 14.5 mm long, and the widest part about 5.5 mm wide. Dark spot on the posterior area of forewings about 1.7 mm wide, and a lighter horseshoe stripe surrounds the dark spot about 2.6 mm wide. Hind wing about 11.3 mm long. Abdomen about 8.7 mm long, and the widest part about 6.2 mm wide. Femora all about 2.0 mm long; left pro- and mesofemur about 0.8 mm wide; right pro- and mesofemur about 0.5 mm wide. Pro- and mesotibiae both about 2.7 mm long. Metatibiae about 4.9 mm long. Right protarsus about 0.9 mm long; preserved part of left protarsus about 0.6 mm long. Mesotarsi about 1.1 mm long. First hind tarsomere about 1.1 mm long; second hind tarsomere about 0.7 mm long; third hind tarsomere for each leg about 0.4 and 0.7 mm long.
Description
Head (Figs. 2, 3b). Most part of the head missing. The basal part of the head preserved. Vertex with posterior and lateral margins carinate, separating from frons. The scape of the antenna cylindrical.
Thorax (Figs. 2, 3a, 4b). Pronotum with several longitudinal and transverse wrinkles; hind margin shallowly concave. Mesonotum with 2 lateral carinae visible; the anterior margin of mesonotum overlapped by pronotum; mesonotum with scutellum forming a posteriorly directed triangle. Tegula covers the base of the forewing. Metapleuron with longitudinal carinae.
Legs (Figs. 2, 3c–h). Left pro- and mesofemora flattened. Tibiae with longitudinal carinae. Metatibiae with six apical spines and at least two lateral spines (with one lateral spine weak-preserved, Fig. 3h), elongated relative to the pro- and mesotibiae. Metatarsus tarsomeres deeply incised; for the third metatarsomere of left and right metalegs differs in length, the first metatarsomere almost the same length or slightly shorter than the second metatarsomere combine with the third metatarsomere; first metatarsomere with 2 lateral and 12 median teeth; second metatarsomere with 2 lateral and 10 median teeth. The metatibio-metatarsal formula is . Tarsi of prolegs and mesolegs with curved claw preserved.
Wings (Figs. 2, 4a–d). Forewings shape ovoid, surpassing moderately abdomen, widest part near the apex of the forewings. Forewings dark-colored with a dark spot on the posterior area, surrounded by a lighter horseshoe stripe. The venations of the left and right forewing overlap due to the lateral compression, and both are partly preserved. Claval veins extend to the cubitus posterior (CuP). Clavus slightly exceeds half of the tegmen length. The common stem of subcosta posterior (ScP) + radius (R) + media posterior (MP) leaves the basal cell. Basal cell longer than wide. The costal area with several crossveins. Main veins divide into numerous branches near the apex of the forewings. Longitudinal branch veins subparallel, with several branches bifurcate. Several crossveins between longitudinal veins. Nodal line concave, with apex near of the tegmen length. Hind wings narrow, with several reticular veins on the anterior cubital area.
Abdomen (Figs. 2, 4e and f). Abdomen ovate and pressed with distortion. The tergite I reduced. Gonocoxa VIII about the same length as the tergite VIII. The apex of the anal tube narrow. The combination of gonoplac, gonapophysis VIII and IX subtrapezoidal, comparatively short, about as long as broad. A round structure between gonoplac and tergite IX, probably a part of gonoplac.
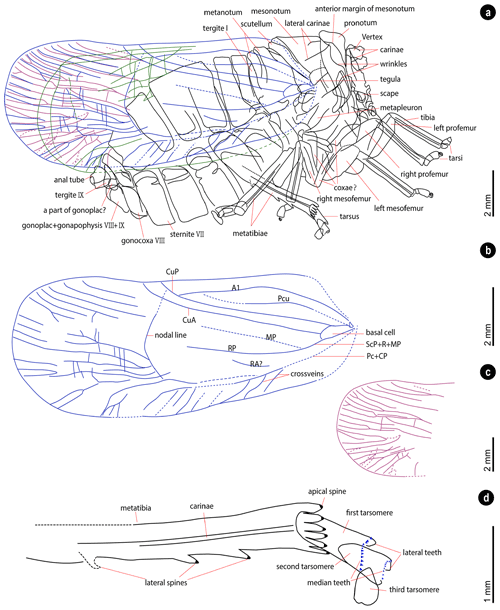
Figure 5Interpretative drawing of the specimen. (a) Illustration of the specimen, veins of the right forewing in blue, veins of the left forewing in red, and veins of the hind wings in green. (b) Venation of the right forewing. (c) Venation of the apex of the left forewing. (d) The tip of the metaleg.
4.1 Systematic assignment
This fossil belongs to the superfamily Fulgoroidea because of a combination of characters, including the presence of tegula, a Y-shaped claval vein in the forewing, two or three strong lateral spines, and a row of apical spines on the hind tibia (Figs. 3–5). Among Auchenorrhyncha, planthoppers morphologically resemble leafhoppers and spittlebugs. Planthoppers can be distinguished by the placement of the eyes, antennae, and lateral ocelli on the gena; the existent of tegula and a Y-shaped claval vein in the forewing; the hind tibia which has several strong lateral spines and rows of spines at the apex (O'Brien, 2008). On the other hand, the hind tibia of leafhoppers has four rows of specialized macrochaetae (Bartlett et al., 2018), and the hind tibia of spittlebugs have one or two stout spines and a single or double row of spines at the apex (Brambila and Hodges, 2008).
The occurrence of Fulgoroidea has been known since the Jurassic, containing 21 extant families (Szwedo et al., 2004). Two basic states of the second metatarsomere, namely pectinate and reduced, are considered important characters in the classification of Fulgoroidea at the family level (Shcherbakov, 2006). Following these states, Fulgoroidea can be divided into two groups – those with a complete row of spines at the apex of the second metatarsomere (Achilidae, Achilixiidae, Cixiidae, Delphacidae, Derbidae, Dictyopharidae, Fulgoridae, Kinnaridae, and Meenoplidae) and those with a pair of spines or without spines at the apex of the second metatarsomere (Issidae, Caliscelidae, Nogodinidae, Acanaloniidae, Flatidae, Tropiduchidae, Tettigometridae, Ricaniidae, Lophopidae, Eurybrachidae, Gengidae, and Hypochthonellidae) (Shcherbakov, 2006). The latter groups are the advanced Fulgoroidea, which are informally regarded as “higher Fulgoroidea”, and the former are basal and intermediate taxa regarded as “lower Fulgoroidea”, but not all phylogenetic studies support this trichotomy (Shcherbakov, 2004; Bartlett et al., 2018).
The fossil in this study belongs to the “lower Fulgoroidea” because of the row of spines at the apex of the second metatarsomere (Figs. 3d–f, 5d). Among the nine families of “lower Fulgoroidea”, Cixiidae and Delphacidae are sister groups, regarded as the early diverging families of Fulgoroidea (Urban and Cryan, 2009; Brysz and Szwedo, 2019), and can be distinguished from our specimen by bearing a sword-shaped ovipositor (Asche, 1987). Kinnaridae and Meenoplidae are sister groups, which are small in body size, with a length less than 10 mm (Brambila and Hodges, 2008), and bear a low density of veins for forewings, distinctly different from our fossil specimen (Figs. 2, 5b and c). Achilixiidae ranges from 4 to 8 mm in length, characterized by one or two pairs of cup-like processes in the lateral abdomen (Liang, 2009), which is not present in our fossil specimen. Achilidae ranges from 3 to 13 mm in length, recognized by the overlapping tips of the forewings (Fennah, 1950), whereas the broad overlap of the apical forewings is not shown in our specimen. Derbidae ranges from 4 to 16 mm in length, frequently fragile, with forewings often much longer than the body (Bartlett et al., 2018), while the forewings of our specimen are oval, slightly longer than the abdomen, and bears a greater number of veins than Derbidae.
After excluding our fossil from seven families from the “lower Fulgoroidea”, the specimen falls within the morphological characters of Fulgoridae and Dictyopharidae, which are sister groups (Asche, 1987; Urban and Cryan, 2007; Song and Liang, 2013). Both families are medium to large planthoppers, and they share high similarity on general structures including chest, wings, legs, abdomen, a fraction of the male genitalia and ovipositor, and both often have an elongated head (Emeljanov, 1979; Song and Liang, 2013). Fulgoridae differs from all other Auchenorrhyncha by the reticulation of the anal area of the hind wings (Muir, 1923). Emeljanov (1979) listed several morphological characters to differentiate these two families. The author pointed out that the hind margin of pronotum in Fulgoridae is usually straight, whereas it is strongly concave in all winged Dictyopharidae; hind femur and tibia in Fulgoridae are distinctly shorter than in Dictyopharidae; three teeth form the inner group for apical teeth of hind tibiae in Dictyopharidae, whereas there are two teeth in Fulgoridae. With regard to the fossil specimen from the Lunpola Basin, a few reticular veins are preserved on the anterior cubital area; the hind margin of pronotum is shallowly concave; fore legs and hind legs relatively short; the inner group for apical teeth of hind tibiae has two teeth, and bears a relatively large density of subparallel apical veins of forewings. Based on all these characters, our fossil specimen is a member of the Fulgoridae.
Fulgoridae contains approximately 142 genera worldwide (Bourgoin, 2021), and the classification at the genus level is mostly based on the shape of the head and the veins of the forewings (e.g., Fennah, 1956; Constant, 2004; Bartlett et al., 2014). However, the specimen studied here lacks most of its head, part of the veins of the forewings and most of the veins of the hind wings. In addition, our specimen bears some peculiar characters compared with the typical representative of modern Fulgoridae, including fewer cross veins on the forewings, the unique horseshoe-shaped pattern of the forewing, the relatively shorter legs, and the peculiar arrangement of teeth on the metatarsomeres. Those peculiarities and the incomplete preservation prevent us from assigning the specimen to an extant or extinct genus but as a new morphotaxon. The accurate systematic attribution can be further defined if more Fulgoridae specimens with head and complete wings are collected.
The fossil records of Fulgoridae are rich and span from the Eocene to the Miocene in the Northern Hemisphere (Wappler, 2003, 2004; Szwedo et al., 2004; Szwedo and Wappler, 2006; Szwedo, 2008). The systematic positions for some of those fossil records have been altered and others are still unresolved (Shcherbakov, 2006). The oldest confirmed fossil assembly of Fulgoridae comes from the early Eocene (ca. 50 Ma) (Brysz and Szwedo, 2019). North American and European fossil records are the richest and mainly from Eocene deposits (Szwedo et al., 2004). Until now, the Asian fossil records only come from the middle Miocene Shanwang Formation in China, including Aphaena lithoecia (Zhang, 1989), Limois shanwangensis (Hong, 1979), L. pardalis (Zhang, 1989), and Ptomatosaiva endea (Zhang et al., 1994). Therefore, our specimen represents the first Paleogene Fulgoridae fossil record in Asia, as well as a new morphotaxon of Fulgoridae.
Fulgoridae usually has colored, opaque, and slightly thickened forewings and patterned hind wings (Bartlett et al., 2018). The lighter horseshoe pattern on the apical forewing of our specimen (Fig. 2) is uncommon in the extant fulgorids. Similar patterns can be found in some living species from Southeast Asia, e.g., Scamandra (Fulgoridae) and Bythopsyrna (Flatidae). Those patterns were assumed to make them inconspicuous against tree trunks or function to startle potential predators because they are similar to the eyes of vertebrate natural predators such as birds (Blest, 1964; Brambila and Hodges, 2008).
4.2 Warm climate and low elevation in the central QTP during the middle Eocene
Modern Fulgoridae is mainly distributed in subtropical and tropical regions, and both adults and nymphs feed on the aboveground parts of their host plants (Brambila and Hodges, 2008). The life cycle of planthoppers is greatly affected by their host plants, and many of them show specific preference for choosing their host plants. The majority of fulgorid species (∼ 81 % of records) feed on dicots (Hamamelidae, Caryophyllidae, Dilleniidae, Rosidae, and Asteridae), while only few species (∼ 16 % of records) feed on monocots (Poaceae and Arecaceae) (Denno and Perfect, 1994). A total of ∼ 62 % of fulgorid species have been reported to feed on a single host, and ∼ 29 % are polyphagous (Denno and Perfect, 1994).
Vegetation in the present fossil site is an alpine meadow with an elevation of ∼ 4655 m, where no Fulgoridae can survive because of the absence of their host plants and the harsh climate condition. The only living species of Fulgoridae recorded in Tibet was Limois chagyabensis from Chaya County, at an elevation of ∼ 3200 m (Zhou and Lu, 1981). Thus, the elevation of the Lunpola Basin should be much lower during the Eocene than nowadays, as highlighted by previous paleontological studies (e.g., Wang and Wu, 2015; Wu et al., 2017; Jiang et al., 2018; Cai et al., 2019; Su et al., 2019).
The new fossil record from the Eocene Lunpola Basin coexisted with some plant species with living relatives mainly in lowland vegetation, including Sabalites (Arecaceae; Su et al., 2019), Koelreuteria (Rosidae; Jiang et al., 2018), Cedrelospermum (Hamamelidae; Jia et al., 2018), Limnobiophyllum (Arecidae; Low et al., 2020), Ailanthus (Rosidae; Liu et al., 2019), Pistacia (Rosidae), Handeliodendron (Rosidae), Exbucklandia (Hamamelidae), Populus (Dilleniidae), Magnoliales (Magnoliidae), and Araliaceae (Rosidae) (Wu et al., 2017), some of which might be potential host plants of Fulgoridae (Denno and Perfect, 1994). In addition to plants, several tropical–subtropical aquatic animal species are found from the same layer, including Eoanabas thibetana (Anabantidae; Wu et al., 2017), Tchunglinius tchangii (Cyprinidae; Wang and Wu, 2015), and Aquarius lunpolaensis (Gerridae; Lin, 1981; Cai et al., 2019). Therefore, as the new evidence from the insects' perspective, our fossil specimen corroborates previous paleoenvironmental assessment that a tropical–subtropical ecosystem was present in the central QTP in the middle Eocene (Wu et al., 2017; Su et al., 2019).
All material included in the paper is deposited in the Paleoecology Collections, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, China.
TS and ZKZ designed the study. XTX wrote a first draft of the manuscript relating to description and taxonomy, took the photographs, and prepared the drawing. WYDD created Fig. 1. TW provided references and revised the taxonomic description. All authors discussed the results and revised the manuscript.
The authors declare that they have no conflict of interest.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank the members of the Paleoecology Research Group in Xishuangbanna Tropical Botanical Garden (XTBG) and Fei-Xiang Wu and his colleagues from Institute of Vertebrate Paleontology and Paleoanthropology for fossil collecting on the Qinghai–Tibetan Plateau; Cédric Del Rio, Jia Liu, and Teng-Xiang Wang for their useful comments, which notably improved the manuscript; Zhi-Shun Song for discussions and for providing references; the Public Technology Service Center and XTBG for help with imaging. We are grateful to Jacek Szwedo and an anonymous reviewer for their helpful comments that improved the clarity and accuracy of the work.
This research has been supported by the National Natural Science Foundation of China (grant nos. 41922010, 41661134049), the Strategic Priority Research Program, CAS (grant nos. XDA20070301, XDB26000000), and the NSFC project Science Center for Tibetan Plateau Earth System (BCTES, grant no. 41988101).
This paper was edited by Carolin Haug and reviewed by Jacek Szwedo and one anonymous referee.
Asche, M.: Preliminary thoughts on the phylogeny of Fulgoromorpha (Homoptera: Auchenorrhyncha), in: Proceedings of the 6th Auchenorrhyncha meeting, Turin, Italy, 7–11 September 1987, 47–53, 1987.
Bartlett, C., O'Brien, L., and Wilson, S.: A review of the Planthoppers (Hemiptera: Fulgoroidea) of the United States, Memoirs of the American Entomological Society, 50, 1–287, 2014.
Bartlett, C. R., Deitz, L. L., Dmitriev, D. A., Sanborn, A. F., Soulier-Perkins, A., and Wallace, M. S.: The Diversity of the True Hoppers (Hemiptera: Auchenorrhyncha), in: Insect Biodiversity, edited by: Foottit, R. G. and Adler, P., Science and Society, 2, 501–590, https://doi.org/10.1002/9781118945582.ch19, 2018.
Blest, A. D.: Protective display and sound production in some New World arctiid and ctenuchid moths, Zoologica, 49, 161–181, 1964.
Bourgoin, T.: Female genitalia in Hemiptera Fulgoromorpha, morphological and phylogenetic data, Ann. Soc. Entomol. Fr., 29, 225–244, 1993.
Bourgoin, T.: Flow (Fulgoromorpha Lists On The Web): A world knowledge base dedicated to fulgoromorpha, Version 8, available at: http://hemiptera-databases.org/flow/, last access: 28 March 2021.
Bourgoin, T., Wang, R.-R., Asche, M., Hoch, H., Soulier-Perkins, A., Stroidski, A., Yap, S., and Szwedo, J.: From micropterism to hyperpterism: recognition strategy and standardized homology-driven terminology of the forewing venation patterns in planthoppers (Hemiptera: Fulgoromorpha), Zoomorphology, 134, 63–77, 2015.
Brambila, J. and Hodges, G. S.: Bugs (Hemiptera), in: Encyclopedia of Entomology (Second Edition), edited by: Capinera, J. L., Springer, New York, 591–611, 2008.
Brysz, A. and Szwedo, J.: Jeweled Achilidae – a new look at their systematics and relation to other Fulgoroidea (Hemiptera), Monographs of the Upper Silesian Museum, 10, 93–130, https://doi.org/10.5281/zenodo.3600279, 2019.
Cai, X. and Fu, J.: Paleocene and Eocene Palaeobiocoenotic feature in the Dazhuoma section at Gangni village of Qiangtang Basin, 33, 443–446, 2003 (in Chinese with English Abstract).
Cai, C.-Y., Huang, D.-Y., Wu, F.-X., Zhao, M., and Wang, N.: Tertiary water striders (Hemiptera, Gerromorpha, Gerridae) from the central Tibetan Plateau and their palaeobiogeographic implications, J. Asian Earth Sci., 175, 121–127, https://doi.org/10.1016/j.jseaes.2017.12.014, 2019.
Chen, S.-S., Shi, R., Gong, X.-H., Liu, D.-L., Huang, Q., Yi, G.-D., Wu, K., and Zou, H.: A syn-collisional model for Early Cretaceous magmatism in the northern and central Lhasa subterranes, Gondwana Res., 41, 93–109, https://doi.org/10.1016/j.gr.2015.04.008, 2017.
Constant, J.: Fulgoridae of Madagascar: description of a new species representing a new genus, key to the genera and list of the species (Homoptera: Fulgoromorpha: Fulgoridae), Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Entomologie, 74, 29–34, 2004.
Denno, R. and Perfect, T.: Planthoppers: their ecology and management, Chapman and Hall, New York, https://doi.org/10.5860/choice.32-3300, 1994.
Dietrich, C. H.: Auchenorrhyncha: (cicadas, spittlebugs, leafhoppers, treehoppers, and planthoppers), in: Encyclopedia of insects, edited by: Resh, V. H. and Cardé, R. T., Academic Press, London, 56–64, https://doi.org/10.1016/B978-0-12-374144-8.00015-1, 2009.
Emeljanov, A. F.: Problema razgranichenhiya semeïstv Fulgoridae i Dictyopharidae (Homoptera: Auchenorrhyncha), Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, 82, 3–22, 1979.
Fang, X., Dupont-Nivet, G., Wang, C., Song, C., Meng, Q., Zhang, W., Nie, J., Zhang, T., Mao, Z., and Chen, Y.: Revised chronology of central Tibet uplift (Lunpola Basin), Sci. Adv., 6, eaba7298, https://doi.org/10.1126/sciadv.aba7298, 2020.
Farnsworth, A., Lunt, D., Robinson, S., Valdes, P., Roberts, W., Clift, P. D., Markwick, P., Su, T., Wrobel, N. E., Bragg, F., Kelland, S. J., and Pancost, R.: Past East Asian monsoon evolution controlled by paleogeography, not CO2, Sci. Adv., 5, eaax1697, https://doi.org/10.1126/sciadv.aax1697, 2019.
Fennah, R. G.: A generic revision of Achilidae (Homoptera; Fulgoroidea) with descriptions of new species, Bulletin of the British Museum of Natural History, Entomology, 1, 1–170, https://doi.org/10.5962/BHL.PART.27228, 1950.
Fennah, R. G.: Fulgoroidea from southern China, Proceedings of the California Academy of Sciences, 28, 512–514, 1956.
Harris, N.: The elevation history of the Tibetan Plateau and its implications for the Asian monsoon, Palaeogeogr. Palaeocl., 241, 4–15, https://doi.org/10.1016/j.palaeo.2006.07.009, 2006.
Hong, Y.: Oxycephala gen. nov., A Miocene Homoptera (Insecta) from Linqu of Shandong, Acta Paleontologica Sinica, 18, 301–307, 1979.
Jarvis, A., Reuter, H. I., Nelson, A., and Guevara, E.: Guevara, Hole-filled seamless SRTM data V4, International Centre for Tropical Agriculture (CIAT), available at: http://srtm.csi.cgiar.org (last update: November 2018), 2008.
Jia, L.-B, Su, T., Huang, Y., Wu, F.-X., Deng, T., and Zhou, Z.-K.: First fossil record of Cedrelospermum (Ulmaceae) from the Qinghai-Tibetan Plateau: Implications for morphological evolution and biogeography, J. Syst. Evol., 57, 94–104, https://doi.org/10.1111/jse.12435, 2018.
Jiang, H., Su, T., Wong, W. O., Wu, F., Huang, J., and Shi, G.: Oligocene Koelreuteria (Sapindaceae) from the Lunpola Basin in central Tibet and its implication for early diversification of the genus, J. Asian Earth Sci., 175, 99–108, 2018.
Li, Y., Liu, X., Ren, D., Li, X., and Yao, Y.: First report of Cixiidae insect fossils from the Miocene of the northeastern Tibetan Plateau and their palaeoenvironmental implications, Alcheringa: An Australasian Journal of Palaeontology, 41, 54–60, https://doi.org/10.1080/03115518.2016.1180027, 2017.
Liang, A.: Morphology of antennal sensilla in Achilixius sandakanensis Muir (Hemiptera: Fulgoromorpha: Achilixiidae) with comments on the phylogenetic position of the Achilixiidae, Raffles B. Zool., 49, 221–225, https://doi.org/10.1016/J.JSEAES.2018.01.014, 2009.
Lin, Q.-B.: Two new species of Tertiary insect fossils from northern Xizang, in: Paleontology of Xizang, edited by: Qinghai-Xizang Plateau Expedition Group, Science Press, Beijing, 345–348, 1981 (Chinese with English abstract).
Lin, Q.-B. and Huang, D.-Y.: Discovery of Paleocene Prophalangopsidae (Insecta, Orthoptera) in the Jiangtang Basin, Northern Tibet, China, Alcheringa: An Australasian Journal of Palaeontology, 30, 97–102, https://doi.org/10.1080/03115510608619346, 2006.
Liu, J., Su, T., Spicer, R. A., Tang, H., Deng, W.-Y.-D., Wu, F., Srivastava, G., Spicer, T. E., Do, T. V., Deng, T., and Zhou, Z.-K.: Biotic interchange through lowlands of Tibetan Plateau suture zones during Paleogene, Palaeogeogr. Palaeocl., 524, 33–40, https://doi.org/10.1016/J.PALAEO.2019.02.022, 2019.
Low, S. L., Su, T., Spicer, T. E. V., Wu, F.-X, Deng, T., Xing, Y.-W., and Zhou, Z.-K: Oligocene Limnobiophyllum (Araceae) from the central Tibetan Plateau and its evolutionary and palaeoenvironmental implications, J. Syst. Palaeontol., 18, 415–431, https://doi.org/10.1080/14772019.2019.1611673, 2020.
Martynov, A. V.: Permian fossil insects from the Arkhangelsk district, Trudy Paleontologicheskogo Instituta Akademii Nauk SSSR, Transactions of the Paleontological Institute, Academy of Sciences, USSR, 4, 1–35, 1935.
Muir, F.: On the classification of the Fulgoroidea (Homptera), Proceeding of the Hawaian Entomological Society, 5, 205–247, 1923.
Nault, L., Rodríguez, J., and Delong, D. M. (Eds.): The leafhoppers and planthoppers, John Wiley and Sons, New York, 1985.
O'Brien, L. B.: Planthoppers (Hemiptera: Fulgoroidea), in: Encyclopedia of Entomology (Second Edition), edited by: Capinera, J. L., Springer, New York, 2922–2930, 2008.
Shao, Z.-G., Meng, X.-G., Zhu, D.-G., Yang, C.-B., Lei, W.-Z., Wang, J., Han, J.-E., Yu, J., Meng, Q.-W., and Qian, C.: Discovery and significance of insect fossils in the Quaternary Kongma basin, Naqu, Tibet, China, Geological Bulletin of China, 29, 254–258, 2010 (in Chinese with English Abstract).
Shcherbakov, D. E.: On Permian and Mesozoic Fulgoroidea, in: Third European Hemiptera Congress, St. Petersburg, Russia, 8–11 June 2004, 68–70, 2004.
Shcherbakov, D. E.: The earliest find of Tropiduchidae (Homoptera: Auchenorrhyncha), representing a new tribe, from the Eocene of Green River, USA, with notes on the fossil record of higher Fulgoroidea, Russian Entomological Journal, 15, 315–322, 2006.
Song, N. and Liang, A.: A Preliminary molecular phylogeny of planthoppers (Hemiptera: Fulgoroidea) based on nuclear and mitochondrial DNA sequences, PLoS ONE, 8, e58400, https://doi.org/10.1371/journal.pone.0058400, 2013.
Spicer, R. A.: Tibet, the Himalaya, Asian monsoons and biodiversity−in what ways are they related, Plant Diversity, 39, 233–244, https://doi.org/10.1016/j.pld.2017.09.001, 2017.
Su, T., Farnsworth, A., Spicer, R. A., Huang, J., Wu, F., Liu, J., Li, S., Xing, Y.-W., Huang, Y., and Deng, W.: No high Tibetan Plateau until the Neogene, Science Advances, 5, eaav2189, https://doi.org/10.1126/sciadv.aav2189, 2019.
Sun, J.-M., Xu, Q.-H., Liu, W.-M., Zhang, Z.-Q., Xue, L., and Zhao, P.: Palynological evidence for the latest Oligocene – early Miocene paleoelevation estimate in the Lunpola Basin, central Tibet, Palaeogeogr. Palaeocl., 399, 21–30, https://doi.org/10.1016/j.palaeo.2014.02.004, 2014.
Sun, T., Wang, C.-S., Li, Y.-L., and Wei, Y.-S.: Characteristics and significance of sedimentary organic matter in the Paleogene of Lunpola basin, central Tibet, Geochimica, 41, 530–537, 2012 (in Chinese with English Abstract).
Szwedo, J.: A new tribe of Dictyopharidae planthoppers from Eocene Baltic amber (Hemiptera: Fulgoromorpha: Fulgoroidea), with a brief review of the fossil record of the family, Palaeodiversity, 1, 75–85, 2008.
Szwedo, J.: The unity, diversity and conformity of bugs (Hemiptera) through time, Earth Env. Sci. T. R. So., 107, 109–128, https://doi.org/10.1017/S175569101700038X, 2018.
Szwedo, J. and Wappler, T.: New Planthoppers (Insecta: Hemiptera: Fulgoromorpha) from the Middle Eocene Messel Maar, Ann. Zool., 56, 555–566, 2006.
Szwedo, J., Bourgoin, T., and Lefebvre, F.: Fossils Planthoppers (Hemiptera: Fulgoromorpha) of the world: An annotated catalogue with notes on Hemiptera classification, Museum and Institute of Zoology, Polish Academy of Sciences, Studio, Poland, 199 pp., 2004.
Szwedo, J., Stroiński, A., and Lin, Q.-B.: Discovery of a Flatidae planthopper (Hemiptera: Fulgoromorpha) in the Paleocene of Northern Tibet and its taxonomic and biogeographic significance, Geodiversitas, 35, 767–776, https://doi.org/10.5252/g2013n4a2, 2013.
Szwedo, J., Stroiński, A., and Lin, Q.: Tip of the clade on the top of the World−the first fossil Lophopidae (Hemiptera: Fulgoromorpha) from the Palaeocene of Tibet, Sci. Nat., 102, 5–6, https://doi.org/10.1007/s00114-015-1277-4, 2015.
Urban, J. M. and Cryan, J. R.: Evolution of the planthoppers (Insecta: Hemiptera: Fulgoroidea), Mol. Phylogenet. Evol., 42, 556–572, https://doi.org/10.1016/J.YMPEV.2006.08.009, 2007.
Urban, J. M. and Cryan, J. R.: Entomologically famous, evolutionarily unexplored: The first phylogeny of the lanternfly family Fulgoridae (Insecta: Hemiptera: Fulgoroidea), Mol. Phylogenet. Evol., 50, 471–484, https://doi.org/10.1016/j.ympev.2008.12.004, 2009.
Wang, H., Fang, Y., Li, S., Hou, X.-O., Wang, B., and Zhang, H.-C.: Revisiting of the Paleocene orthopteran insect Hylophalangopsis chinensis Lin and Huang, 2006 in Northern Tibet, J. Asian Earth Sci., 175, 93–98, https://doi.org/10.1016/j.jseaes.2018.06.003, 2019.
Wang, N. and Wu, F.-X.: New Oligocene cyprinid in the central Tibetan Plateau documents the pre-uplift tropical lowlands, Ichthyol. Res., 62, 274–285, https://doi.org/10.1007/s10228-014-0438-3, 2015.
Wappler, T.: Die Insekten aus dem Mittel-Eozän des Eckfelder Maares, Vulkaneifel, Mainzer naturwissenschaftliches Archiv, Beiheft, 27, 1–234, 2003.
Wappler, T.: Notes on a plant-hopper (Hemiptera: Fulgoromorpha: Dictyopharidae) from the Middle Eocene Messel Maar, Germany, Neues Jahrb. Geol. P. M., 2004, 694–704, https://doi.org/10.1127/njgpm/2004/2004/694, 2004.
Wei, W., Lu, Y., Xing, F., Liu, Z., Pan, L., and Algeo, T. J.: Sedimentary facies associations and sequence stratigraphy of source and reservoir rocks of the lacustrine Eocene Niubao Formation (Lunpola Basin, central Tibet), Mar. Petrol. Geol., 86, 1273–1290, https://doi.org/10.1016/j.marpetgeo.2017.07.032, 2017.
Wu, F., Miao, D., Chang, M., Shi, G., and Wang, N.: Fossil climbing perch and associated plant megafossils indicate a warm and wet central Tibet during the late Oligocene, Sci. Rep., 7, 1–7, https://doi.org/10.1038/s41598-017-00928-9, 2017.
Yang, Q., Shi, C., Li, X., Pang, H., and Ren, D.: The first fossil brown lacewing from the Miocene of the Tibetan Plateau (Neuroptera, Hemerobiidae), ZooKeys, 726, 145–154, https://doi.org/10.3897/zookeys.726.21086, 2018.
Yao, T., Thompson, L. G., Mosbrugger, V., Zhang, F., Ma Ma, Y., Luo, T., Xu, B., Yang, X., Joswiak, D. R., Wang, W., Joswiak, M. E., Devkota, L. P., Tayal, S., Jilani, R., and Fayziev, R.: Third pole environment (TPE), Enviro. Dev., 3, 52–64, https://doi.org/10.1016/j.envdev.2012.04.002, 2012.
Yao, T.-D., Chen, F.-H., Cui, P., Ma, Y.-M., Xu, B.-Q., Zhu, L.-P., Zhang, F., Wang, W.-C., Ai, L., and Yang, X.: From Tibetan plateau to third pole and pan-third pole, Bulletin of Chinese Academy of Sciences, 32, 924–931, 2017 (in Chinese with English Abstract).
Zhang, J.: Fossil insects from Shanwang, Shandong, China, Shandong Science and Technology Press, Jinan, 126 pp., 1989 (in Chinese with English Abstract).
Zhang, J., Sun, B., and Zhang, X.: Miocene insects and spiders from Shanwang, Shandong, Science Press, Beijing, 298 pp., 1994 (in Chinese with English Abstract).
Zhou, Y. and Lu, J.: Fulgoroidea, in: Insects of Xizang, vol.I, edited by: Qinghai-Xizang Plateau Expedition Group, Chinese Academy of Sciences, Beijing, 221–232, 1981 (in Chinese with English Abstract).




