the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Past ecosystems drive the evolution of the early diverged Symphyta (Hymenoptera: Xyelidae) since the earliest Eocene
Arvid Aase
André Nel
Paleoxyela nearctica gen. et sp. nov., is described from the upper Eocene of Florissant Formation in Colorado. We placed Paleoxyela gen. nov. in the subfamily Macroxyelinae and the tribe Macroxyelini based on the numerous wing venation characters visible on the specimen. Proxyelia pankowskii gen. et sp. nov. is described from the lower Eocene Fossil Lake deposits of the Green River Formation in Wyoming. We placed Proxyelia gen. nov. in the subfamily Macroxyelinae and the tribe Xyeleciini based on the numerous wing venation characters visible on the specimen. These new records of the family Xyelidae are of particular importance to better understand the past diversity of the clade and propose hypotheses about their diversification. Extant Xyelidae inhabit temperate Northern Hemisphere forests, and most of their larvae feed on conifers, which may explain why they are relatively poorly diversified compared to the other symphytan families. We suggest that the global decline in conifers and the reduced diversity of extant host trees partly explain the diversity of extant Xyelidae. We correlate the biome repartition during the Eocene to that of the extant xyelid.
The “symphytan” superfamily Xyeloidea Newman, 1834, is broadly accepted as the earliest diverging clade within the crown group Hymenoptera (Ronquist et al., 2012), while a recent study rather suggested the Pamphilioidea as the earliest diverging clade (Peters et al., 2017). Therefore, documenting the fossil record of this superfamily is crucial to better understand the early radiation of Hymenoptera and refine the time divergence estimate of the Hymenoptera arising. The Xyeloidea currently comprise the Xyelidae Newman, 1834, and the †Syspastoxyelidae Engel and Huang, 2016 (Zheng et al., 2021a). The former comprises about 80 extant species distributed in five genera (Taeger et al., 2018). Interestingly, the fossil record of the Xyelidae is abundant, even if rather poorly documented. More than 80 Mesozoic species within 47 genera are currently attributed to Xyelidae (e.g., Rasnitsyn, 1964, 1969; Wang et al., 2012; Kopylov, 2014; Zheng et al., 2019a, 2021b). In comparison, the Cenozoic record of the family is more reduced, with only 14 species known to date (Table 1).
If the extant Xyelidae are widely distributed in the Northern Hemisphere, they clearly display a relict distribution in view of their past distribution and diversity, i.e., known from deposits of the Southern Hemisphere (e.g., Lara et al., 2014) and from numerous species. This distribution may be partially explained by the distribution of the host plants of their larvae. Extant xyelid larvae are phytophagous and associated with trees. Some xyeline species are associated with conifers, which may explain the reduced number of extant species (see discussion below). Interestingly, conifers are also known from Fossil Lake but are limited to a winged seed, dubious cone structures, and groups of needles which are more likely attributed to disarticulated Ceratophyllum leaves.
Here we describe two new North American fossils belonging to the crown group of the family Xyelidae, from the Ypresian Green River Formation and from the Chadronian Florissant Fossil Beds.
The Fossil Lake sediments of the Green River Formation are dated from ca. 51.98 Ma (Smith et al., 2008, 2010), corresponding to the Early Eocene Climatic Optimum (EECO), a long-lived hypothermal event which may have had an impact on the evolutionary history of the Xyelidae. The Florissant Fossil Beds are younger than the Green River Formation since they are dated ca. 34.07 Ma (Evanoff et al., 2001) and slightly postdate another important thermal event of the Eocene: Middle Eocene Climatic Optimum (MECO).
The discovery of these new xyelid genera in the Florissant Fossil Beds and in the Green River Formation, together with the distribution of Mesozoic and extant xyelid, suggests that the family was already widely distributed at this latitude during the Eocene. Note that the Eocene Fushunoxyela viridocapitata (Hong, 2002) is not treated as a xyelid wasp (Blank et al., 2009, p. 31). We correlate the discoveries of Paleoxyela nearctica gen. et sp. nov. and Proxyelia pankowskii gen. et sp. nov. and the past biome heterogeneity, particularly that of the Eocene period, to assume that the Eocene biomes have participated in conditioning the distribution of extant Xyelidae.
2.1 Florissant fossil beds
The holotype of Paleoxyela nearctica gen. et sp. nov. derives from Florissant Fossil Beds National Monument in Teller County, Colorado (see Smith, 2008, fig. 1). The Florissant Formation has been dated at 34.07 Ma (Evanoff et al., 2001), and the fossil beds were deposited in a paleolake that was formed by the volcanoclastic debris flow damming of the drainage basin (McLeroy and Anderson, 1966; Evanoff et al., 2001). The study of the fossil plant assemblage suggests a warm–temperate paleoenvironment (MacGinitie, 1953). More recent studies, using several proxies (physiognomic and floristic criteria: e.g., Meyer, 2001; Leopold and Clay-Poole, 2001; multivariate analysis of leaf characters: e.g., Gregory and McIntosh, 1996; comparison to modern forest communities: Boyle et al., 2008; and the mutual climate range of Diptera: Moe and Smith, 2005), have estimated mean annual temperatures (MATs) between 10.8 and 17.5 ∘C (today between 9.30 and 18.97 ∘C). Additional study also quantified on-site precipitation at approximately 50 cm per year using the composition of the fossil flora (MacGinitie, 1953).
The holotype of Paleoxyela nearctica gen. et sp. nov. is housed in the collection of the Museum of Natural History, University of Colorado, USA, under the collection number UCM 18643. Photographs have been taken with a Canon EOS 5Ds camera equipped with 65 mm MPE and 100 mm canon macro lenses. A StackShot macro rail was used to acquire the images that are digitally stacked photomicrographic composites of several individual focal planes obtained using Helicon Focus 6.7.
2.2 Green River Formation
The geology of the Green River Formation is relatively well understood. This formation is composed of lacustrine sediments deposited in three basins by intermittently interconnected lakes over a span of 8 million years (Smith et al., 2008 and others cited therein). The holotype specimen of Proxyelia pankowskii gen. et sp. nov. originates from the vicinity of Clear Creek Valley (10 mi, or 16.09 km, west of Kemmerer, Wyoming, USA) in a talus block dislodged from the cliff above the 18 in. (45.72 cm) layer and below the K-spar tuff (Archibald et al., 2021, fig. 2). The K-spar tuff, dated at 51.98 Ma, provides the only radiometric date for the Fossil Lake deposits of the Green River Formation (Smith et al., 2008, 2010). Association with Lambdotherium popoagicum (Brontotheriidae, Perissodactyla), known from the 18 in. (45.72 cm) layer, denotes a Wasatchian 7 North American land mammal age. The holotype of Proxyelia pankowskii gen. et sp. nov. is housed in the collection of Fossil Butte National Monument, Wyoming, USA, under the collection number FOBU14313. Photographs have been taken with a FUJI GFX 50S camera. The figures were composed with Adobe Illustrator CC2019 and Photoshop CC2019 software.
Wing venation terminology is adapted from Goulet and Hubert (1993). Published work and nomenclatural acts are registered in ZooBank (http://zoobank.org/, last access: 5 November 2021), with the following life science identifier (LSID) (reference): urn:lsid:zoobank.org:pub:578A65E0-4FE8-4852-A840-AAC4FAA2A5F7.
-
Order: Hymenoptera Linnaeus, 1758
-
Suborder: Symphyta Gerstaecker, 1867
-
Superfamily: Xyeloidea Newman, 1834
-
Family: Xyelidae Newman, 1834
-
Subfamily: Macroxyelinae Ashmead, 1898
-
Tribe: Macroxyelini Ashmead 1898
-
Paleoxyela gen. nov.
urn:lsid:zoobank.org:act:7F15690A-0437-4F67-AF19-D9FFE1F3CD18
Etymology
The generic name is a combination of the prefix paleo- and xyelia commonly used for the xyelid genera.
Type species
Paleoxyela nearctica sp. nov.
Diagnosis
Antenna with eight flagellomeres distad first elongate flagellomere; forewing with vein Sc2 joining R far from separation of Rs from R, 1-RS and 1-M nearly equal in length; short ovipositor; legs short (i.e., coxa not reaching abdomen mid-length and legs not 1.5–2.0 × as long as body).
-
Paleoxyela nearctica sp. nov.
(Figs. 1–3)
urn:lsid:zoobank.org:act:71648E38-0501-49B6-AE0D-970C3C5B7DE1

Figure 1Photograph of Paleoxyela nearctica gen. et sp. nov. Holotype UCM 18643. Scale bar: 5 mm. (© Talia Karim).

Figure 2Paleoxyela nearctica gen. et sp. nov. Holotype UCM 18643. (A) Detailed photograph of head and thorax. (B) Detailed photograph of right wings. (C) Detailed photograph of left wings. Scale bars: 1 mm. (© Talia Karim).
Holotype
Specimen identifier UCM 18643 housed in the collection of the Museum of Natural History (University of Colorado, USA).
Etymology
The specific epithet refers to the Nearctic origin of the fossil and is to be treated as an adjective.
Locality and age
Florissant, Colorado, Paleogene, Eocene, Chadronian.
Diagnosis
As for the genus (vide supra).
Description
Head transverse 1.71 mm long (mandibles included) and 2.32 mm wide, vertex broadly rounded; eyes wide, occupying a large part of head lateral surface; mandibles projected anteriorly, partly covered by labrum; ocelli not preserved; four conspicuous antennomeres (apical part poorly visible and exact number not countable); scape ca. 0.50 mm long; first flagellomere ca. 3.06 mm long, slightly wider near mid-length, remaining part of flagellum ca. 0.23 mm long (maybe longer but not preserved).
Thorax slightly wider than head, pronotum (in dorsal view) short dorsomedially (ca. 0.35 mm long), slightly longer laterally, mesoscutum massive, with notauli impressed but apparently not meeting posteriorly, mesoscutellum, and remaining part of the thorax not discernable.
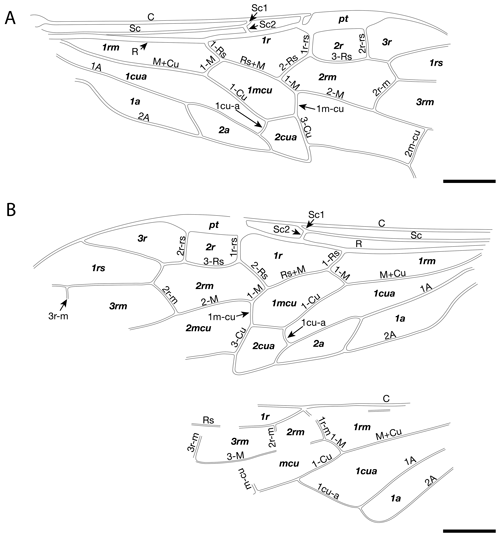
Forewing ca. 9.93 mm long and 3.16 mm wide. Pterostigma sclerotized, wide and longer than cell 2r; costal area not dilated proximad near Sc1 and Sc2 fork; Sc with two branches, Sc1 meeting with C far beyond Rs, subequal to Sc2; Sc2 almost vertical, meeting R near the middle of cell 1rs; Sc2 conspicuously shorter than 1-Rs, the latter subequal to 1-M; R slightly curved before origin of Rs and slightly thickened before pterostigma; costal space apically with vertical sclerotization between C with R delimited from pterostigma with costal break (membraneous slit); crossveins 1r-rs and 2r-rs, equal in length, straight or nearly so; Rs+M about 2.3 times as long as 1-Rs; section of Rs between 1r-rs and 2r-rs strongly arched toward posterior margin of forewing; cell 1rs elongate, longer than cell 3r, slightly trapezoidal; M+Cu slightly curved in its basal third; 1m-cu 0.72 times as long as 2-Cu or 3-Cu; 2m-cu straight and 0.41 times as long as 4-Cu.
Hind wing with 1r-m long, meeting Rs slightly distad its base; cell 1r thin and elongate; 1-M fully preserved, apparently shorter than 1r-m; 2r-m and 3r-m straight, both nearly equal in length; M+Cu straight; m-cu meeting 3-M in the middle of 3r; 1cu-a not fully preserved, meeting Cu in the middle of cell mcu; cell 1cua broad and long; 1A nearly straight; cell 1a elongate, rectangular; 2A complete, nearly straight.
Legs partly preserved. Hind femur ca. 3.20 mm long, slightly wider medially; hind tibia at least as long as femur.
Abdomen with 10 visible segments, widest near mid-length, length of abdominal segments measured dorsomedially (in millimeters): 0.53, 1.02, 0.89, 0.88, 0.90, 0.82, 0.94, 0.70, 0.56 (IX and X combined). Ovipositor ca. 0.75 mm long, cercus slightly protruding from abdomen posteriorly from segment 10.
Remark
To date, there is only one Macroxyelini fossil species known from Florissant – Megaxyela petrefacta (Brues, 1908; Zhelochovtzev and Rasnitsyn, 1972) – but the new specimen clearly differs from the latter species owing to the different configuration of the vein 1-RS and 1-M, i.e., almost equal in length (vs. 1-M much shorter than 1-RS in Megaxyela).
-
Tribe: Xyeleciini Benson, 1945
-
Proxyelia gen. nov.
urn:lsid:zoobank.org:act:3A8D4BFC-5BF2-47E3-81E5-3758AD8D273A
Etymology
The generic name is a combination of the prefix pro- and xyelia commonly used for the xyelid genera.
Type species
Proxyelia pankowskii sp. nov.
Diagnosis
Flagellum short (less than 2 times longer than head); forewing with pterostigma completely sclerotized; costal area sclerotized apically (near pterostigmal base); small desclerotization present near base of pterostigma; Sc two-branched, with posterior branch (Sc2) shorter than anterior branch (Sc1); Sc2 slightly inclined, aligned with and meeting 1-Rs in a point; Sc1 longer than 1-Rs; 1-Rs distinct, conspicuously shorter than 1-M; crossvein 1r-rs subvertical, slightly shorter than 2r-rs; cell 1rs short (not elongate); 1m-cu short, about 3 times shorter than 3-Cu; cell 1mcu long and narrow, 3.7 times as long as wide. Third antennomere subequal to head length.
-
Proxyelia pankowskii sp. nov.
(Figs. 4–5)
urn:lsid:zoobank.org:act:E7E6CD5E-3AEB-4427-8260-32EC0C49803D
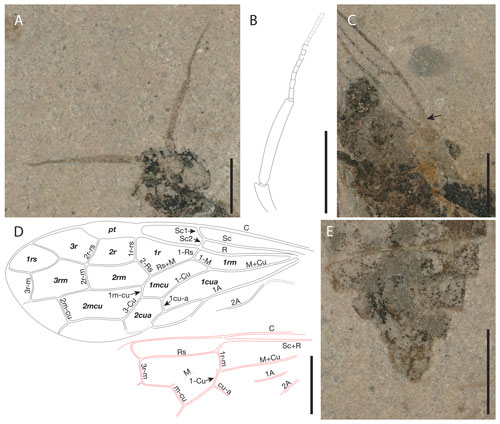
Figure 5Proxyelia pankowskii gen. et sp. nov. Holotype FOBU14313. (A) Detailed photograph of head. (B) Interpretative line drawing of antenna. (C) Detailed photograph of pterostigma. (D) Interpretative line drawing of venation with cell (in bold) and vein names labeled. (E) Detailed view of apical part of abdomen. Scale bars: 1 mm.
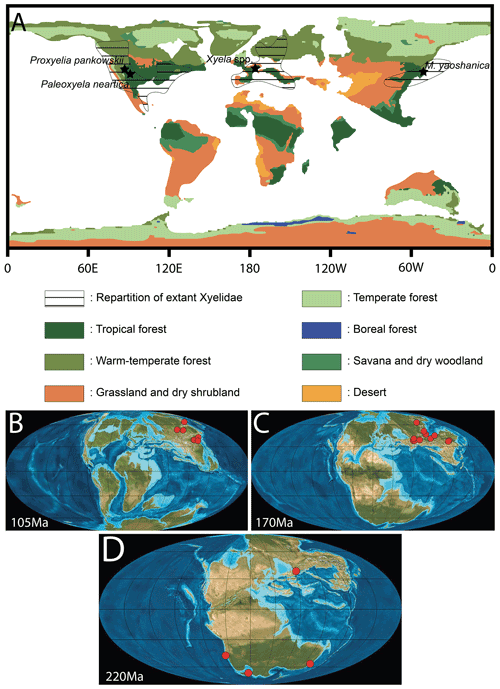
Figure 6(A) Map of the Eocene world with vegetation simulated by BIOME4 (modified from Herold et al., 2014) and distribution of extant Xyelidae (from https://v3.boldsystems.org, last access: 7 November 2021). (B) World map of the Cretaceous period at 105 Ma with xyelid localities indicated. (C) World map of the Jurassic period at 170 Ma with xyelid localities indicated. (D) World map of the Triassic period at 220 Ma with xyelid localities indicated (B, C, D; © PALEOMAP project).
Holotype
Specimen identifier FOBU14313, housed in the collection of Fossil Butte National Monument, Wyoming, USA.
Etymology
Named after Michael Pankowski, who, after recognizing the importance of the fossil offered for sale on the internet, purchased and donated the specimen to Fossil Butte National Monument. The specific epithet is to be treated as a noun in a genitive case.
Locality and age
Clear Creek Valley, 10 mi (16.09 km) west of Kemmerer, Wyoming, USA; Ypresian, lowermost Eocene (ca. 51.98 Ma).
Diagnosis
As for the genus (vide supra).
Description
Head rounded, 1.15 mm long (mandibles included) and 1.31 mm wide; eyes wide, occupying a large part of head lateral surface; mandibles projected anteriorly, partly covered by labrum; ocelli not preserved; 12 conspicuous antennomeres (apical part poorly visible and exact number not countable); scape ca. 0.32 mm long; first flagellomere ca. 1.05 mm long, slightly wider near mid-length, remaining part of flagellum ca. 1.14 mm long; posterior margin of head concave.
Thorax slightly wider than head ca. 1.38 mm wide.
Forewing ca. 5.46 mm long and 1.90 mm wide. Pterostigma sclerotized, wide, and longer than cell 2r; costal area obviously dilated proximad near Sc1 and Sc2 fork; Sc with two branches, Sc1 meeting with C slightly distad of Rs, longer than Sc2; Sc2 almost vertical, meeting R before at the originating point of 1-Rs; Sc2 slightly shorter than 1-Rs, the latter distinctly shorter than 1-M; R strongly curved before origin of Rs and slightly thickened before pterostigma; crossvein 1r-rs straight; 2r-rs slightly bend near Rs and ca. 0.8 times as long as 1r-rs; distance between base of 2r-rs and 1r-rs nearly twice as long as distance between apex of pterostigma and base of 2r-rs; Rs+M about 4.2 times as long as 1-Rs; section of Rs between 1r-rs and 2r-rs slightly arched toward posterior margin of forewing; cell 1rs short, slightly trapezoidal; M+Cu slightly curved medially; 1m-cu about half as long as 2-Cu and about 0.34 as long as 3-Cu; 2m-cu straight and 0.49 times as long as 4-Cu.
Hind wing with 1r-m long, meeting Rs slightly distad its base; 1-M not fully preserved, ca. 2.7 times longer than 1r-m; m-cu not reaching 3r-m, separated from 3r-m base (along M) by a distance nearly equal to 3r-m length; cu-a not fully preserved; M and Cu with free ends apparently not reaching wing margin; 1A with not fully preserved, slightly curved; 2A not fully preserved.
Legs partly preserved. Foreleg thin and short, not clearly discernible, with tibia ca. 0.84, mm long. Mid femur at least 0.91 mm long; tibia 0.92 mm; tarsi ca. 1.22 mm long, with four preserved tarsomeres, first tarsomere the longest; length of tarsomeres from base to apex (in millimeters): 0.52, 0.23, 0.22, 0.22. Hind femur ca. 1.20 mm long, slightly wider medially; tibia ca. 1.67 mm long and ca. 0.19 mm at maximal width; tarsi ca. 1.34 mm long, with four preserved tarsomeres, basitarsomere the longest (ca. 0.59 mm long); length of other tarsomeres from base to apex (in millimeters): TII 0.26, TIII 0.19, TIV 0.23.
Abdomen with at least nine visible segments, widest near mid-length.
Systematic placement
Following the key to extant hymenopteran families of Mason (1993), Paleoxyela gen. nov. and Proxyelia gen. nov. key out in the family Xyelidae because of the following couplets: forewings well developed; body apparently lacking constriction between the thorax and abdomen, these elements being largely related to each other; wing venation complete, with a pterostigma and several closed cells, especially on hind wing; mesoscutum touching scutellum over a long oblique groove on each side. Forewing with longitudinal vein Sc between C and R; extremely elongated first flagellomere, more than 5 times longer than its width and significantly longer than subsequent flagellomeres.
The character “enlarged first flagellomere succeeded by a series of thinner and shorter flagellomeres” is present in Xyelidae but also the three Mesozoic families Syspastoxyelidae; Xyelotomidae Rasnitsyn, 1968; and Xyelydidae (Engel et al., 2016). The mid-Cretaceous family Syspastoxyelidae differs from Paleoxyela gen. nov. and Proxyelia gen. nov. in the reduced venation in the distal part of the forewing (Engel et al., 2016; Wang et al., 2019; Zheng et al., 2019b, 2021a). The Mesozoic Xyelotomidae have a distinctly broader forewing cell 1mcu and a very short 1-Rs (Gao et al., 2009, 2016). Wang et al. (2016) recovered the Xyelydidae as paraphyletic within the Pamphiloidea; nevertheless they also have a forewing cell 1mcu broader than in Paleoxyela gen. nov. and Proxyelia gen. nov. (Rasnitsyn, 1969, 1983; Wang et al., 2015).
Following the key to subfamilies and tribes of Xyelidae proposed by Zheng et al. (2021b), Paleoxyela gen. nov. keys out in the subfamily Macroxyelinae and in the tribe Macroxyelini because of the following characters: along R, cell 2r shorter than or, very rarely, as long as 1r; costal space apical with vertical sclerotization between C with R delimited from pterostigma with costal break; pterostigma sclerotized (dark); ovipositor not narrow, extending behind abdomen; Sc hind branch entering R distal of Rs base; 1-Rs not distinctly shorter than 1-M; area around costal break narrowly or not at all desclerotized; first flagellomere not shorter than posterior flagellomeres together. Currently, the tribe Macroxyelini encompasses two genera, Macroxyela and Megaxyela. The new specimen cannot be placed in the genus Macroxyela owing to the vein Sc2 of forewing joining R far from the separation of Rs from R (vs. close in Macroxyela) but also due to the presence of eight flagellomeres after the first elongate flagellomere. The new specimen differs from representatives of the genus Megaxyela owing to the forewing with vein 1-RS and 1-M nearly equal in length (vs. 1-M much shorter than 1-RS, usually about half as long as 1-RS in Megaxyela), the short ovipositor (vs. relatively long), the legs not 1.5–2.0 × as long as body, the coxae short (vs. long nearly reaching abdomen mid-length) (Blank et al., 2017b, p. 9), and due to the presence of eight flagellomeres distad the first elongate flagellomere (Shinohara, 1992).
Following the key to subfamilies and tribes of Xyelidae proposed by Zheng et al. (2021b), Proxyelia gen. nov. keys out in the subfamily Macroxyelinae and in the tribe Xyeleciini (originally considered to be a subfamily by Benson, 1945, and treated as a tribe by Smith, 1967) because of the following characters: along R, cell 2r shorter than cell 1r; costal space apically with vertical sclerotization between C with R delimited from pterostigma with costal break (well visible on right wing and indicated by an arrow in Fig. 2C); pterostigma fully sclerotized (dark); posterior branch of Sc entering R at Rs base; 1-Rs about half as long as 1-M.
The tribe Xyeleciini comprises only the extant genus Xyelecia Ross, 1932, and the five Mesozoic genera Xyelites Rasnitsyn, 1966; Uroxyela Rasnitsyn, 1966; Microxyelecia Rasnitsyn, 1969; Bolboxyela Rasnitsyn, 1990; and Scleroxyela Zheng et al., 2021b. Proxyelia gen. nov. differs from Xyelites and Uroxyela at least in having a distinct 1-Rs and a long third antennomere; from Microxyelecia in having a long third antennomere, i.e., as long as head (vs. shorter), the anterior branch of Sc (Sc1) long (vs. short), and a different configuration of wing apex (i.e., with a long cell under 3r); from Xyelecia in having a short third antennomere, i.e., as long as head (vs. longer) and in the forewing a second branch of Sc (Sc2) meeting R opposite base of 1-Rs (vs. well basal 1-Rs); and from Scleroxyela in the same last character. Proxyelia gen. nov. further differs from Xyelecia in the vein 1-Rs emerging from R obliquely (vs. at right angle), cell 1r shorter and distally broader; shorter cell 3r, as long as cell 1r (vs. distinctly longer than 1r); cell 1rs very short (Ross, 1932, pl. 1, fig. 1; Togashi, 1972, fig. 4).
5.1 Decline in conifers may have limited the diversification of Xyelinae
Larvae of numerous extant Xyelinae species feed on conifers or closely related taxa (e.g., Shinohara, 1995; Yates and Smith, 2009), and numerous Xyelidae are also known to be monophagous (Byun et al., 2005; Blank et al., 2013). However, some species are less specific in their host choice and can feed on several host plants (Blank et al., 2017a). In many cases, such specializations prevent hosts from shifting to other tree species and constrain the life of the wasp to that of the host. In a case of a global decline in the conifers, it is expected to observe a stagnation of or a reduction in the species diversity within lineages closely related to these trees. Recently, Condamine et al. (2020) investigated the diversification of conifers during geological times and demonstrated that a shift occurred in the diversification dynamics of conifers, with a decrease in origination and an increase in extinction near the Cretaceous–Paleocene boundary. Therefore, the Xyelidae that were adapted to the conifers that stopped diversifying during this period likely did not survive. Similarly, the overall decline in conifers may have limited the speciation and diversification of the Xyelidae after the Cretaceous period. These assumptions are confirmed by the recent analyses of Nyman et al. (2019) showing that symphytan lineages with larvae feeding on angiosperms (e.g., Tenthredinidae) have diversified rapidly during and after the Cretaceous period (Nyman et al., 2019, figs. 1–2). This rapid diversification of sawfly groups feeding on angiosperms is correlated with the diversification of the angiosperms during the Early Cretaceous (Nyman et al., 2019, fig. 3). It is also assumed that some of these groups have switched to feed on angiosperms during the Early Cretaceous, whereas other lineages (e.g., Xyelidae) retained their ancestral diet, died, or switched much later after the decline in the gymnosperms. Therefore, even if the “host” plants of Mesozoic Xyelidae are unknown, they likely fed on gymnosperms, and the decline in the latter has likely induced the decline in or the stagnation of Xyelidae, while the lineages that have switched to feed on angiosperms have benefited from their diversification and have also diversified.
In the case of the larvae of Macroxyelinae, which are generally free feeders on deciduous tree species (e.g., on Ulmaceae and/or Juglandaceae) (Smith and Schiff, 1998; Blank et al., 2017b), the reduced diversity of these families may explain why they are today relatively little diversified compared to the Tenthredinidae for example. The extant Ulmaceae include about 45 species (Christenhusz and Byng, 2016), which are widely distributed throughout the north temperate zone, corresponding to the distribution of Macroxyelinae. Similarly, the extant Juglandaceae include about 50 species (Christenhusz and Byng, 2016) in the Americas, Eurasia, and Southeast Asia, also corresponding to the distribution of Macroxyelinae. Even if the Macroxyelinae seem to be less specific in their host choices, the reduced number of potential host plant species is maybe one explanation for their current diversity and distribution.
The extant genus Xyelecia comprises only the two species, X. nearctica Ross, 1932, from western North America and X. japonica Togashi, 1972, from Japan. Nothing seems to be known about their biology. Only Smith (1967, p. 377) indicated that they are “probably shoot borers in fir”. Pinus are recorded from the Green River Formation (Wodehouse, 1933; note that the pollen is from Lake Uinta, deposited ca. 3 Myr after Fossil Lake), suggesting a possible similar biology for Proxyelia gen. nov., but Ulmaceae and Juglandaceae are also present in the Green River paleobiota (Smith, 2008).
The plant–insect relationships occurring between the Xyelidae and their host plants provide some clues to explain their current reduced diversity. However, an analysis of the diversification for the whole “Symphyta”, allowing the correlation of the diversification pattern to a series of variables (temperature, humidity, flora, land bridges, etc.), should clarify these assumptions.
5.2 Biome heterogeneity and repartition of extant Xyelidae
The Green River Formation is slightly posterior to the Paleocene–Eocene thermal maximum (PETM) and to the major cooling event of the PETM (Wing et al., 2005). However, it corresponds with the Early Eocene Climate Optimum (EECO) (∼ 53.3–49.70 Ma), an episode of global warming characterized by the warmest sustained temperatures of the Cenozoic (e.g., Zachos et al., 2001, 2008). Herold et al. (2014) presented a general biome model for the Eocene, based on several simulated vegetation distribution and topographic reconstructions, and recovered the presence of “paratropical” forests in North America (ca. 30∘ N) and across Eurasia at the same latitude. However, the model indicated a large area of drought in the middle paleo-Eurasian continent, forming a barrier between European and Southeast Asian rainforest expansions (Fig. 6A). In addition, the broad seas and oceans have certainly played a role in limiting xyelid's intercontinental migrations. Therefore, the localities of the known Eocene, Oligocene, and Miocene xyelids were located within these Eocene paratropical forests (Fig. 6) or in warm–temperate and subtropical forests, relictual from the Eocene period. The record of two representatives of the family in the same biome from two deposits very distant may suggest that the family lived in this type of forest during the early Eocene, the EECO, and the subsequent Eocene hyperthermals (Willard et al., 2019).
Interestingly, there is no record of fossil Xyelidae or of extant xyelid in the desertic area (comprising deserts, grasslands, and dry shrub lands) located between western Europe and eastern Asia (Fig. 6). Therefore, the past biome configuration probably led to the current xyelid distribution. We assume that the arid areas, recorded during the Eocene, have played a role as climate barriers between Europe and Asia, firstly preventing spreading or faunal exchanges between the two continents and secondly constraining the repartition of Asian and European Eocene Xyelidae to the past outer zone of paratropical and subtropical forests. The fossil record of the family after the Eocene, particularly during the Oligocene and the Miocene, seems to corroborate this hypothesis since it is only known from the Oligocene Rott formation in Germany (Fig. 6A; see also Meunier, 1920; Benson, 1960; Rasnitsyn, 1995) and from the Miocene Shanwang Formation in China (Zhang, 1989), both warm–temperate. In fact, these fossil representatives are present in areas corresponding to the previous paratropical forest or the outer zone of the latter of the Eocene. The distribution of biomes from the Eocene may have led to the restricted distribution of Xyelidae. The EECO may have had an impact which is difficult to quantify on the diversification of Xyelidae. The fact that temperatures increased overall during this period may have reinforced the barrier effect of arid biomes present between Europe and Asia.
During the Mesozoic, the xyelid wasps were relatively broadly distributed, with representatives found mainly on land masses in the Northern Hemisphere (Fig. 6B, C, D; data compiled from http://fossilworks.org/, last access: 7 November 2021). During the Cretaceous the family is recorded in Eurasia in several deposits from China and Russia (Fig. 6B). This restricted distribution seems to be corroborated by the study of numerous Lagerstätten (e.g., Burmese amber, Crato Formation) in which the family Xyelidae is not recorded. In Burmese amber, the family Syspastoxyelidae (also belonging to the Xyeloidea) is found and appears to be quite diversified (Engel et al., 2016; Wang et al., 2019). The reduced wing venation and the combination of characters showed by the Syspastoxyelidae may be the result of an evolution in insular condition of a xyeloid lineage. If this assumption is later verified, it may be indicative of a Gondwana xyeloid lineage that colonized the West Burma block prior to its separation from southern Gondwana. However, this hypothesis is highly speculative given the fact that no Xyelidae are recorded in Gondwana during the Jurassic or the Cretaceous (e.g., Brothers and Rasnitsyn, 2003). In the Crato formation, there is no record of the family Xyelidae despite the numerous fossils of Hymenoptera known to date. However, it is important to keep in mind that the fossil record is partial, which may also explain the restricted distribution of the family during the Cretaceous. Similarly, during the Jurassic, the Xyelidae seems very well established in eastern Laurasia (Fig. 6C) but absent from the Southern Hemisphere. It was only during the Triassic that the Xyelidae displayed a global distribution in the northern and southern hemispheres (Fig. 6D), with representatives of the stem Xyelidae (Lara et al., 2014). Deep comparison with the biome repartition at each geological period may partially explain this distribution but is beyond the scope of the present paper.
The most striking point is, as mentioned above, that the distribution of extant xyelid wasps seems to be correlated with their distribution during the Cenozoic and constrained by the geological and environmental barriers of the time. Nevertheless, the insect Cenozoic record in the eastern part of Europe and the Middle East is very fragmentary. Future discoveries may challenge the present hypothesis.
The descriptions of Paleoxyela nearctica gen. et sp. nov. and Proxyelia pankowskii gen. et sp. nov. help to increase the knowledge of the diversity of the family Xyelidae during the Cenozoic. The peculiar biology of extant xyelid wasps (i.e., larvae feeding on conifers) may have been an obstacle to their diversification since the diversity of their hosts has decreased over time in favor of a dominance of angiosperms. Therefore, lineages that have retained the ancestral feeding habits of the group (i.e., feeding on gymnosperms) have not diversified, while the sawfly groups that switched their diet to feed on angiosperms have greatly diversified in parallel with the angiosperm diversification. Interestingly, the repartition of the Eocene biomes is closely related to the current distribution of Xyelidae and maintained during the Oligocene. We therefore assume that there could be a correlation between these two variables and that the distribution of biomes during the Eocene played a major role in the evolutionary history of the clade after the Cretaceous.
All material included in the paper is deposited in the collection of Fossil Butte National Monument, Wyoming, USA, and the collection of the Museum of Natural History (University of Colorado, USA).
CJ and AN designed the study. CJ wrote a first draft of the manuscript relating to description and taxonomy. AA took the photographs of FOBU14313 and drafted text related to the geology of Fossil Lake. CJ prepared the drawing. AN provided references and revised the taxonomic description. All authors discussed the results and revised the manuscript.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We give our sincere thanks to Michael Pankowski for his time scouring the internet looking for scientifically significant fossils being offered for sale. Without his effort and generosity (and support) this paper would not be possible. We are grateful to Talia Karim (University of Colorado Museum of Natural History) for the photographs of Paleoxyela nearctica gen. et sp. nov. (Specimen UCM 18643). We warmly thank the two reviewers Alexandr P. Rasnitsyn and Lars Vilhelmsen for their constructive and useful comments on the early version of the manuscript. We also thank Florian Witzmann for managing the article during the editing process. Text and images by Arvid Aase were completed as part of his official duties and as such are considered to be a US Government publication. Under provisions of the Copyright Act (17 USC Section 8) there is no copyright claimed on any of his text or images.
This paper was edited by Florian Witzmann and reviewed by Alexandr P. Rasnitsyn and Lars Vilhelmsen.
Archibald, S. B., Aase, A., and Nel, A.: The second North American fossil horntail wood-wasp (Hymenoptera: Siricidae), from the early Eocene Green River Formation, Zootaxa, 4999, 325–334, https://doi.org/10.11646/zootaxa.4999.4.2, 2021.
Ashmead, W. H.: Classification of horntails and sawflies (Nematinae) + (Oryssinae) + (Xyelidae) + (Lydidae) + (Hylotominae), Can. Entomol., 30, 141–145 + 281–287 + 177–184 + 205–213, 1898.
Benson, R. B.: Classification of the Xyelidae (Symphyta), P. Roy. Entomol. Soc. B, 14, 34–37, https://doi.org/10.1111/j.1365-3113.1945.tb00013.x, 1945.
Benson, R. B.: Two new European species of Xyela Dalman (Hymenoptera: Xyelidae), P. Roy. Entomol. Soc. B, 29, 110–112, https://doi.org/10.1111/j.1365-3113.1960.tb01153.x, 1960.
Blank, S. M., Taeger, A., Liston, A. D., Smith, D. R., Rasnitsyn, A. P., Shinohara, A., Heidemaa, M., and Viitasaari, M.: Studies toward a World Catalog of Symphyta (Hymenoptera), Zootaxa, 2254, 1–96, https://doi.org/10.11646/zootaxa.2254.1.1, 2009.
Blank, S. M., Shinohara, A., and Altenhofer, E.: The Eurasian species of Xyela (Hymenoptera, Xyelidae): taxonomy, host plants and distribution, Zootaxa, 3629, 1–106, https://doi.org/10.11646/zootaxa.3629.1.1, 2013.
Blank, S. M., Kramp, K., and Shinohara, A.: Xyela fusca spec. nov. from Japan elucidates East Asian–North American relationships of Xyela (Hymenoptera, Xyelidae), Zootaxa, 4303, 103–121, https://doi.org/10.11646/zootaxa.4303.1.6, 2017a.
Blank, S. M., Kramp, K., Smith, D. R., Sundukov, Y. N., Wei, M., and Shinohara, A.: Big and beautiful: the Megaxyela species (Hymenoptera, Xyelidae) of East Asia and North America, Eur. J. Taxon., 348, 1–46, https://doi.org/10.5852/ejt.2017.348, 2017b.
Boyle, B., Meyer, H. W., Enquist, B., and Salas, S.: Higher taxa as paleoecological and paleoclimatic indicators: A search for the modern analog of the Florissant fossil flora, in: Paleontology of the upper Eocene Florissant Formation, Colorado, edited by: Meyer, H. W. and Smith, D. M., Geological Society of America Special Paper, 435 pp., https://doi.org/10.1130/2008.2435(03), 2008.
Brébisson, A.: Sur un nouveau genre d'insectes, de l'ordre des Hyménoptères, Bulletin des Sciences, par la Société Philomatique de Paris, 1818, 116–117, 1818.
Brothers, D. J. and Rasnitsyn, A. P.: Diversity of Hymenoptera and other insects in the Late Cretaceous (Turonian) deposits at Orapa, Botswana: a preliminary review, Afr. Entomol., 11, 221–226, 2003.
Brues, C. T.: New Phytophagous Hymenoptera from the Tertiary of Florissant, Colorado, Bulletin of the Museum of Comparative Zoology, 51, 259–276, https://doi.org/10.5962/bhl.title.19923, 1908.
Byun, B.-K., Blank, S. M., and Shinohara, A.: The East Asian Xyela species (Hymenoptera: Xyelidae) associated with Japanese red pine (Pinus densiflora; Pinaceae) and their distribution history, Insect Systematics and Evolution, 36, 259–278, https://doi.org/10.1163/187631205788838393, 2005.
Condamine, F. L., Silvestro, D., Koppelhus, E. B., and Antonelli, A.: The rise of angiosperms pushed conifers to decline during global cooling, P. Natl. Acad. Sci. USA, 46, 28867–28875, https://doi.org/10.1073/pnas.2005571117. 2020.
Christenhusz, M. J. M. and Byng, J. W.: The number of known plants species in the world and its annual increase, Phytotaxa, 261, 201–217, https://doi.org/10.11646/PHYTOTAXA.261.3.1. 2016.
Engel, M. S., Huang, D.-Y., Alqarni, A. S., and Cai, C.-Y.: An unusual new lineage of sawflies (Hymenoptera) in upper Cretaceous amber from northern Myanmar, Cretaceous Res., 60, 281–286, https://doi.org/10.1016/j.cretres.2015.12.014, 2016.
Evanoff, E., McIntosh, W. C., and Murphey, P. C.: Stratigraphic summary and geochronology of the Florissant Formation, Colorado, 1–16, in: Fossil flora and stratigraphy of the Florissant Formation, Colorado, edited by: Evanoff, E., Gregory-Wodzicki, K. M., and Johnson, K. R., Proceedings of the Denver Museum of Nature and Science, 4th series, 2001.
Gao, T.-P., Ren, D., and Shih, C.-K.: The first Xyelotomidae (Hymenoptera) from the Middle Jurassic in China, Ann. Entomol. Soc. Am., 102, 588–596, https://doi.org/10.1603/008.102.0402, 2009.
Gao, T.-P., Shih, C.-K., Engel, M. S., and Ren, D.: A new xyelotomid (Hymenoptera) from the Middle Jurassic of China displaying enigmatic venational asymmetry, BMC Evol. Biol., 16, 155, https://doi.org/10.1186/s12862-016-0730-0, 2016.
Gerstaecker, C. E. A.: Über die Gattung Oxybelus Latr. und die bei Berlin vorkommenden Arten derselben, Zeitschrift fur die Gesammten Naturwissenschaft, 30, 1–96, 1867.
Goulet, H. and Huber, J. T. (Eds.): Hymenoptera of the world: An identification guide to families. Agriculture Canada, Ottawa, Ontario, 680 pp., 1993.
Gregory, K. M. and McIntosh, W. C.: Paleoclimate and paleoelevation of the Oligocene Pitch-Pinnacle flora, Sawatch Range, Colorado, Geol. Soc. Am. Bull., 108, 545–561, https://doi.org/10.1130/0016-7606(1996)108<0545:PAPOTO>2.3.CO;2, 1996.
Herold, N., Buzan, J., Seton, M., Goldner, A., Green, J. A. M., Müller, R. D., Markwick, P., and Huber, M.: A suite of early Eocene (∼ 55 Ma) climate model boundary conditions, Geosci. Model Dev., 7, 2077–2090, https://doi.org/10.5194/gmd-7-2077-2014, 2014.
Hong, Y.-C.: Fossil insects in the diatoms of Shanwang, Bulletin of the Tianjin Institute of Geology and Mineral Resources, 8, 1–15, 1983.
Hong, Y.-C.: Amber insects of China, Huayu Nature Book Trade Co. Ltd, Henan Scientific and technological publishing house, Henan, 1–653, 2002.
Kopylov, D. S.: New sawflies of the subfamily Madygellinae (Hymenoptera, Xyelidae) from the Middle-Upper Triassic of Kyrgyzstan, Paleontol. J., 48, 610–620, https://doi.org/10.1134/S0031030114060070, 2014.
Lara, M. B., Rasnitsyn, A. P., and Zavattieri, A. M.: Potrerilloxyela menendezi gen. et sp. nov. from the Late Triassic of Argentina: the oldest representative of Xyelidae (Hymenoptera: Symphyta) for Americas, Paleontol. J., 48, 182–190, https://doi.org/10.1134/S0031030114020075, 2014.
Leopold, E. B. and Clay-Poole, S. T.: Florissant leaf and pollen floras of Colorado compared: climatic implications, 17–69, in: Fossil flora and stratigraphy of the Florissant Formation, Colorado, edited by: Evanoff, E., GregoryWodzicki, K. M., and Johnson, K. R., Proceedings of the Denver Museum of Nature and Science, 4th series, 2001.
MacGinitie, H. D.: Fossil plants of the Florissant beds, Colorado, Carnegie Institution of Washington Contributions to Paleontology, 599, 198 pp. 1953.
Mason, W. R. M.: Chapter 5. Key to superfamilies of the Hymenoptera, 65–100, in: Hymenoptera of the World: an identification guide to families, edited by: Goulet, H. and Huber, J. T., Research Branch Agriculture Canada Publication, Ottawa, 1894/E, i–vii + 1–668, 1993.
McLeroy, C. A. and Anderson, R. Y.: Laminations of the Oligocene Florissant lake deposits, Colorado, Geol. Soc. Am. Bull., 77, 605–618, https://doi.org/10.1130/0016-7606(1966)77[605:LOTOFL]2.0.CO;2, 1966.
Meunier, F.: Quelques insectes de l'Aquitanien de Rott, Sept-Monts (Prusse rhénane), Verslagen der Koninklijke Akademie van Wetenschappen te Amsterdam, 22, 891–898, 1920.
Meyer, H. W.: A review of the paleoelevation estimates for the Florissant flora, Colorado, 205–216, in: Fossil flora and stratigraphy of the Florissant Formation, Colorado, edited by: Evanoff, E., Gregory-Wodzicki, K. M., and Johnson, K. R., Proceedings of the Denver Museum of Nature and Science, 4th series, 2001.
Moe, A. P. and Smith, D. M.: Using pre-Quaternary Diptera as indicators of paleoclimate, Palaeogeogr. Palaeocl., 221, 203–214, https://doi.org/10.1016/j.palaeo.2005.02.012, 2005.
Newman, E.: Art. XXXVII. – Attempted division of British insects into natural orders, Entomological Magazine, 2, 379–431, 1834.
Nyman, T., Onstein, R. E., Silvestro, D., Wutke, S., Taeger, A., Wahlberg, N., Blank, S. M., and Malm, T.: The early wasp plucks the flower: disparate extant diversity of sawfly superfamilies (Hymenoptera: “Symphyta”) may reflect asynchronous switching to angiosperm hosts, Biol. J. Linn. Soc., 128, 1–19, https://doi.org/10.1093/biolinnean/blz071, 2019
Peters, R., Krogmann, L., Mayer, C., Donath, A., Gunkel, S., Meusemann, K., Kozlov, A., Podsiadlowski, L., Petersen, M., Lanfear, R., Diez, P., Heraty, J., Kjer, K., Klopfstein, S., Meier, R., Polidori, C., Schmitt, T., Liu, S., Zhou, X., and Niehuis, O.: Evolutionary history of the Hymenoptera, Curr. Biol., 27, 1013–1018, 2017.
Rasnitsyn, A. P.: New Triassic Hymenoptera from Central Asia, Paleontol. Zh., 1964, 88–96, 1964 (in Russian).
Rasnitsyn, A. P.: New Xyelidae (Hymenoptera) from the Mesozoic of Asia, Paleontol. Zh., 1966, 69–85, 1966 (in Russian, translated into English in International Geology Review, 1967, 723–737).
Rasnitsyn, A. P.: Origin and evolution of Lower Hymenoptera, Trudy Paleontologicheskogo Instituta Academii Nauk SSSR, 123, 1–196, 1969 (in Russian).
Rasnitsyn, A. P.: Fossil Hymenoptera of the superfamily Pamphilioidea, Paleontol. J., 17, 56–70, 1983.
Rasnitsyn, A. P.: Tertiary sawflies of the tribe Xyelini (Insecta: Vespida=Hymenoptera: Xyelidae) and their relationship to the Mesozoic and modern faunas, Contributions in Science, Natural History Museum of Los Angeles County, 450, 1–14, 1995.
Ronquist, F., Klopfstein, S., Vilhelmsen, L., Schulmeister, S., Murray, D. L., and Rasnitsyn, A. P.: A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera, Syst. Biol., 61, 973–999, https://doi.org/10.1093/sysbio/sys058, 2012.
Ross, H. H.: The hymenopterous family Xyelidae in North America, Ann. Entomol. Soc. Am., 25, 153–169, https://doi.org/10.1093/aesa/25.1.153. 1932.
Shinohara, A.: The sawfly genus Megaxyela (Hymenoptera, Xyelidae) in East Asia, Japanese Journal of Entomology, 60, 783–796, 1992.
Shinohara, A.: The sawfly genus Pleroneura (Hymenoptera, Xyelidae) in East Asia, Japanese Journal of Entomology, 63, 825–840, 1995.
Smith, D. M.: A comparison of plant-insect associations in the middle Eocene Green River Formation and the Upper Eocene Florissant Formation and their climatic implications, Geol. S. Am. S., 435, 89–103, https://doi.org/10.1130/2008.2435(06) 2008.
Smith, D. R.: A review of the larvae of Xyelidae, with notes on the family classification (Hymenoptera), Ann. Entomol. Soc. Am., 60, 376–384, https://doi.org/10.1093/aesa/60.2.376, 1967.
Smith, D. R. and Schiff, N. M.: The genera Macroxyela Kirby and Megaxyela Ashmead (Hymenoptera: Xyelidae) in North America, P. Entomol. Soc. Wash., 100, 636–657, 1998.
Smith, M. E., Carroll, A. R., and Singer, B.: Synoptic reconstruction of a major ancient lake system: Eocene Green River Formation, western United States, Geol. Soc. Am. Bull., 120, 54–84, https://doi.org/10.1130/B26073.1, 2008.
Smith, M. E., Chamberlain, K. R., Singer, B. S., and Carroll, A. R.: Eocene clocks agree: Coeval , U-Pb, and astronomical ages from the Green River Formation, Geology, 38, 527–530, https://doi.org/10.1130/G30630.1, 2010.
Statz, G.: Über alte und neue fossile Hymenopterenfunde aus den Tertiaren Ablagerungen von Rott am Siebengebirge, Decheniana, 93, 256–312, 1936.
Taeger, A., Liston, A. D., Prous, M., Groll, E. K., Gehroldt, T., and Blank, S. M.: ECatSym – Electronic World Catalog of Symphyta (Insecta, Hymenoptera). Program version 5.0 (19 Dec 2018), data version 40 (23 Sep 2018), Senckenberg Deutsches Entomologisches Institut (SDEI), Müncheberg, available at: https://sdei.de/ecatsym/ (last access: 19 July 2021), 2018.
Togashi, I.: Discovery of the genus Xyelecia (Hymenoptera Xyelidae) from Japan, Kontyû, 40, 87–89, 1972.
von Linnaeus, C.: Systema Naturae per regna tria naturae secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Ed. decima reformata. Holmiae, Laur. Salvii, Typis Ioannis Thomae nob. De Trattnern, Vindobonae, 1, 1–823, 1758.
Wang, M.-X., Shih, C.-K., and Ren, D.: Platyxyela gen. nov. (Hymenoptera, Xyelidae, Macroxyelinae) from the Middle Jurassic of China, Zootaxa, 3456, 82–588, https://doi.org/10.11646/zootaxa.3456.1.4, 2012.
Wang, M.-X., Rasnitsyn, A. P., Shih, C.-K., and Ren, D.: New xyelydid sawflies from the Lower Cretaceous of China, Cretaceous Res., 54, 169–178, https://doi.org/10.1016/j.cretres.2014.12.008, 2015.
Wang, M.-X., Rasnitsyn, A. P., Li, H., Shih, C.-K., Sharkey, M. J., and Ren, D.: Phylogenetic analyses elucidate the inter-relationships of Pamphilioidea (Hymenoptera, Symphyta), Cladistics, 32, 239–260, https://doi.org/10.1111/cla.12129, 2016.
Wang, Y.-M., Lin, X., Wang, M.-D., Shih, C.-K., Ren, D., and Gao, T.-P.: New sawflies from the mid-Cretaceous Myanmar amber (Insecta: Hymenoptera: Syspastoxyelidae), Hist. Biol., 1877, 1–10, https://doi.org/10.1080/08912963.2019.1687695, 2019.
Willard, D. A., Donders, T. H., Reichgelt, T., Greenwood, D. R., Sangiorgi, F., Peterse, F., Nierop, K. G. J., Frieling, J., Schouten, S., and Sluijs, A.: Arctic vegetation, temperature, and hydrology during Early Eocene transient global warming events, Global Planet. Change, 178, 139–152, https://doi.org/10.1016/j.gloplacha.2019.04.012, 2019.
Wing, S. L., Harrington, G. J., Smith, F. A., Bloch, J. I., Boyer, D. M., and Freeman, K. H.: Transient floral change and rapid global warming at the Paleocene-Eocene boundary, Science, 310, 993–996, https://doi.org/10.1126/science.1116913, 2005.
Wodehouse, R. P.: Tertiary pollen-II. The oil shales of the Eocene Green River formation, B. Torrey Bot. Club, 60, 479–524, 1933.
Yates III, H. O. and Smith, D. R.: History, distribution, damage, and life cycle of a pine shoot gall sawfly, Xyela gallicaulis (Hymenoptera: Xyelidae), J. Entomol. Sci., 44, 276–283, 2009.
Zachos, J. C., Pagani, M., Sloan, L., Thomas, E., and Billups, K.: Trends, rhythms, and aberrations in global climate 65 Ma to present, Science, 292, 686–693, https://doi.org/10.1126/science.1059412, 2001.
Zachos, J. C., Dickens, G. R., and Zeebe, R. E.: An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics, Nature, 451, 279–283, https://doi.org/10.1038/nature06588, 2008.
Zhang, J.-F.: Fossil insects from Shanwang, Shandong, China, Shandong Science and Technology Publishing House, 1–459, 1989.
Zhelochovtzev, A. N. and Rasnitsyn, A. P.: On some Tertiary sawflies (Hymenoptera, Symphyta) from Colorado, Psyche, 79, 315–327, https://doi.org/10.1155/1972/63630, 1972.
Zheng, Y., Chen, J., Zhang, J.-Q., and Zhang, H.-C.: New fossil sawflies (Hymenoptera, Xyelidae) from the Middle Jurassic of northeastern China, Alcheringa, 44, 115–120, https://doi.org/10.1080/03115518.2019.1641618, 2019a.
Zheng, Y., Zhang, Q., Chen, J., and Zhang, H.-C.: A remarkably new basal wasp with uniquely transformed forewing in mid-Cretaceous Burmese amber (Hymenoptera, Syspastoxyelidae), Cretaceous Res., 104, 104172, https://doi.org/10.1016/j.cretres.2019.07.002, 2019b.
Zheng, Y., Hu, H., Zhang, H.-C., Chen, J., Rasnitsyn, A. P., and Zhuo, D.: New genus and species of syspastoxyelid sawflies (Insecta, Hymenoptera) from the mid-Cretaceous Kachin amber with a review of the family Syspastoxyelidae, Cretaceous Res., 127, 104940, https://doi.org/10.1016/j.cretres.2021.104940, 2021a.
Zheng, Y., Hu, H.-Y., Chen, D., Chen, J., Zhang, H.-C., and Rasnitsyn, A. P.: New fossil records of Xyelidae (Hymenoptera) from the Middle Jurassic of Inner Mongolia, China, Eur. J. Taxon., 684, 146–159, https://doi.org/10.5852/ejt.2021.733.1229, 2021b.