the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Osteology of the Permian temnospondyl amphibian Glanochthon lellbachae and its relationships
Rainer R. Schoch
The early Permian Meisenheim Formation of the Saar–Nahe Basin (Germany) is famous for its richness in vertebrate fossils, among which the temnospondyls were present with microvores and fish-eating apex predators. The latter trophic guild was occupied exclusively by the genus Sclerocephalus in that basin within a long time interval up to M8, whereas in M9, a new taxon, Glanochthon lellbachae, appeared. This taxon is defined by (1) a preorbital region 1.8–2.0 times as long as the postorbital skull table, (2) dermal ornament with tall radial ridges, (3) a prefrontal anteriorly wider with straight lateral margin, (4) a squamosal posteriorly only half as wide as the quadratojugal, (5) phalanges of manus and pes long and gracile, (6) carpals unossified in adults, and (7) tail substantially longer than skull and trunk combined. Phylogenetic analysis finds that G. lellbachae forms the basal sister taxon of the stratigraphically younger G. angusta and G. latirostre and that this clade nests within the paraphyletic taxon Sclerocephalus, with S. nobilis forming the sister taxon of the genus Glanochthon (urn:lsid:zoobank.org:act:3038F794-17B9-4FCA-B241-CCC3F4423651; registration date: 15 March 2021).
The Saar–Nahe Basin ranks among the largest late Paleozoic sedimentary basins in continental Europe and has produced thousands of early Permian tetrapod fossils (Boy et al., 2012; Fig. 1). The largest tetrapod taxa in these lacustrine deposits were eryopiform temnospondyls, which formed the top predatory group that preyed on bony fishes (Boy, 2003). Among these, the most common genus, Sclerocephalus, evidently preferred actinopterygians, whereas the more gracile Glanochthon and Archegosaurus preserve acanthodian skeletons in their intestines (Boy, 1994; Kriwet et al., 2008; Schoch and Witzmann, 2009a). For more than 150 years, the three taxa were the only well-known eryopiforms from the Saar–Nahe Basin despite much collecting in numerous localities. The only exceptions formed two isolated finds, the still enigmatic Palatinerpeton (Boy, 1996) and a fragmentarily known Onchiodon-like eryopid (Schoch and Hampe, 2004).
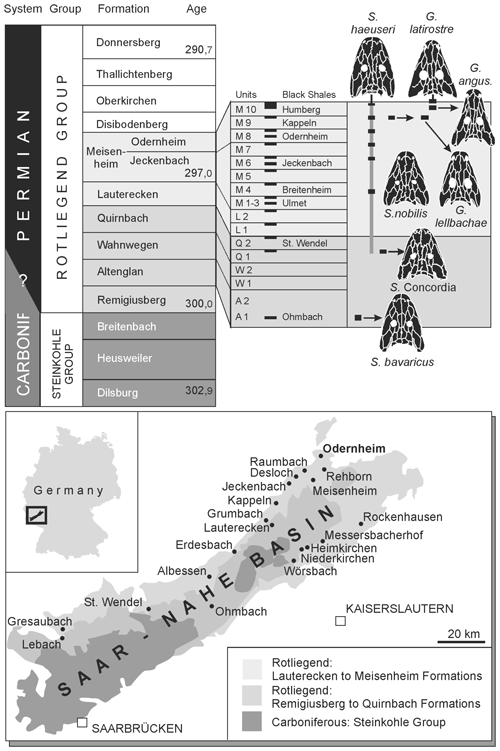
Figure 1Localities yielding Sclerocephalus and Glanochthon material and stratigraphy of the Rotliegend sequence with emphasis on the Meisenheim Formation (Autunian, Lower Permian) in the Saar–Nahe Basin of Germany.
This changed when private collector Klaus Krätschmer discovered a new site in the Klauswald southwest of Odernheim am Glan (Fig. 1), where large quantities of vertebrates were collected. This locality falls within the Meisenheim Formation, the richest tetrapod-bearing rock sequence in the basin (Boy et al., 2012). He published a large part of his material (Krätschmer, 2004, 2006; Krätschmer and Resch, 2005) and compared it with other samples, providing detailed information also on the various localities and fossillagerstätten. His taxonomic considerations led him to suggest the existence of various new taxa, most of which turned out to be synonyms of Sclerocephalus haeuseri Goldfuss (Schoch and Witzmann, 2009a). Nevertheless, his work highlighted the variation between and within samples of eryopiform temnospondyls, and one of the new morphs from the Klauswald locality that he envisioned clearly forms a separate taxon from the co-occurring Sclerocephalus nobilis. This new species was first named Cheliderpeton lellbachae by Krätschmer (2006).
The various samples of Sclerocephalus and Glanochthon span a time range of some 2.5 Myr (Boy et al., 2012; Menning and Bachtadse, 2012), and the morphological and developmental aspects of this endemic evolutionary lineage will be the subject of a research program that seeks to trace the microevolution and cladogenesis between the samples. The present exercise forms only the first step in this program. The objective is to describe and diagnose this taxon, especially in comparison to co-occurring S. nobilis from the same locality and to study its phylogenetic relationships.
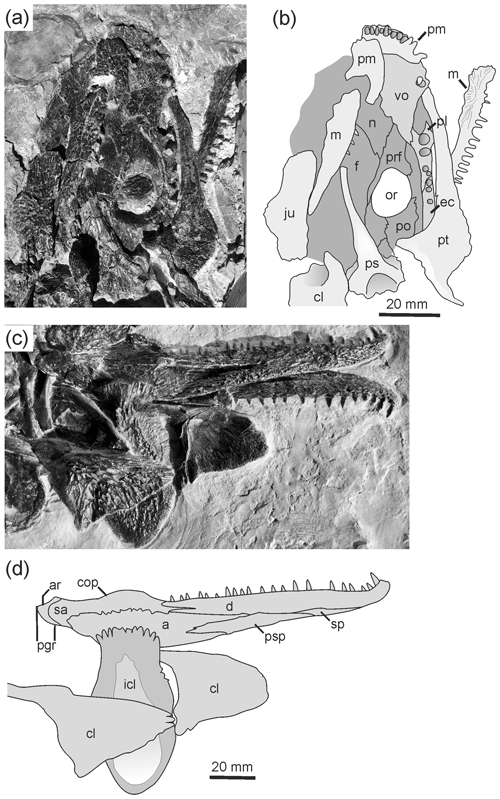
NHMM: Naturhistorisches Museum, Mainz, Germany. NHMM 2006/14 (102 mm SL, SL signifies skull length, complete skeleton with skin preservation; Figs. 2a–c, 3d).
SMNK: Staatliches Museum für Naturkunde, Karlsruhe, Germany. SMNK-TAL 4638a (91 mm SL, complete skeleton); 4638b (91 mm SL, complete skeleton).
SMNS: Staatliches Museum für Naturkunde, Stuttgart, Germany. SMNS 91281 (115 mm SL, complete skeleton; Fig. 3a); 90507 (85 mm SL, palate and postcranium; Figs. 3c, 4c, d).
UGKU: Urweltmuseum Geoskop, Pfalzmuseum für Naturkunde, Thallichtenberg, Germany. UGKU POL-F 1997/1 (Fund-Nr. ROT 658) (115 mm SL, complete skeleton with skin preservation; Figs. 3b, 4a, b).
Anatomical abbreviations
a – angular; ar – articular; cl – clavicle; cop – coronoid process; d – dentary; ec – ectopterygoid; f – frontal; icl – interclavicle; ju – jugal; la – lacrimal; m – maxilla; n – nasal; na – naris; p – parietal; ptf – postfrontal; pgr – postglenoid region; pl – palatine; pm – premaxilla; po – postorbital; pp – postparietal; prf – prefrontal; ps – parasphenoid; psp – postsplenial; pt – pterygoid; q – quadrate; qj – quadratojugal; sa – surangular; sp – splenial; sq – squamosal; st – supratemporal; t – tabular; vo – vomer.
-
Order Temnospondyli Zittel, 1888
-
Rhachitomi Watson, 1919 sensu Schoch, 2013
-
Eryopiformes Schoch, 2013
-
Family Sclerocephalidae Jaekel, 1909
-
Genus Glanochthon Schoch and Witzmann, 2009
Diagnosis
(1) Preorbital region 1.8–2.2 times as long as postorbital skull table, (2) tabular horn prominent, (3) postglenoid region posteriorly longer than the articular facet, (4) jugal narrower than greatest orbit width, and (5) interclavicle slender and at least twice as long as wide (modified from Schoch and Witzmann, 2009b).
Type species
Glanochthon latirostre (Jordan, 1849).
-
Glanochthon lellbachae (Krätschmer, 2006) comb. nov. (Figs. 2–5)
-
Cheliderpeton lellbachae Krätschmer, 2006
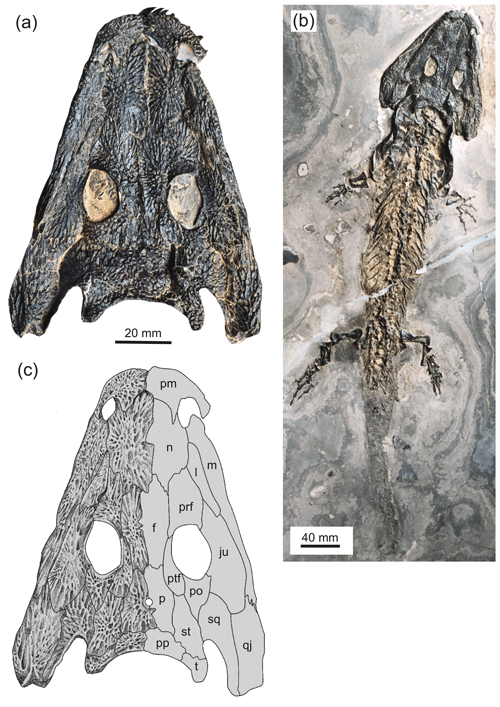
Figure 2Type specimen of Glanochthon lellbachae (Krätschmer, 2006) comb. nov. (NHMM 2006/14). (a, c) Close-up of skull roof and (b) complete skeleton.
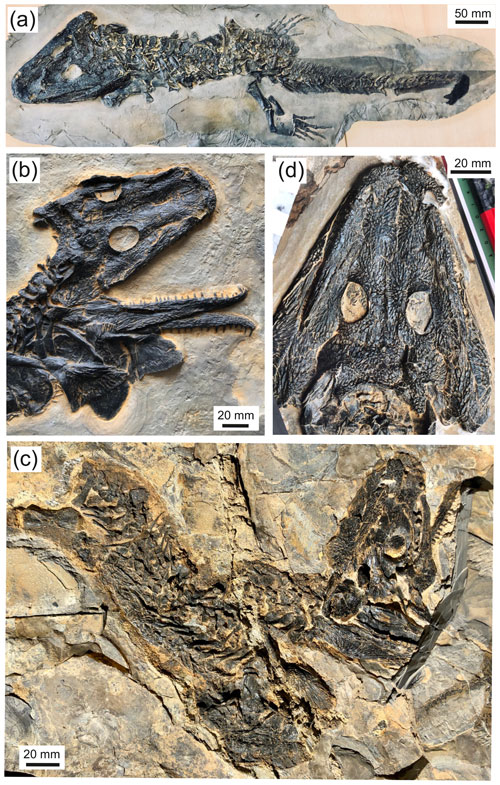
Figure 3Glanochthon lellbachae (Krätschmer, 2006) comb. nov.: (a) SMNS 91281, (b) UGKU POL-F 1997/1, (c) SMNS 90507 and (d) NHMM 2006/14.
Holotype
NHMM 2006/14, 102 mm SL, complete skeleton with skin impression (Fig. 2).
Type of locality and age
The Klauswald southwest of Odernheim am Glan, Rhineland-Palatinate, Germany (Fig. 1). M9 sequence, Klauswald facies, Odernheim Subformation, Meisenheim Formation, lower Rotliegend, Autunian, lowermost Permian.
Referred material
Altogether, five additional specimens are referred to as Glanochthon lellbachae (see material section). There remains a substantial number of specimens in private collections.
Diagnosis
Autapomorphies: (1) preorbital region in adults 1.8–2.0 times as long as postorbital skull table, (2) dermal ornament with continuous and relatively tall radial ridges in the snout, frontals and cheek (contrasting the more polygonal arrangement in S. nobilis), (3) prefrontal anterolaterally expanded to form a more equant pentagon, (4) squamosal posteriorly only half as wide as quadratojugal, (5) phalanges of manus and pes slightly longer and more gracile than in S. nobilis and (6) tail substantially longer than skull and trunk combined (shorter than that measurement in S. nobilis).
Taxonomic assignment
This taxon was originally erected as Cheliderpeton lellbachae by Krätschmer (2006), who made reference to its resemblance to Cheliderpeton latirostre as described and referred to by Boy (1993). Schoch and Witzmann (2009b) suggested the new genus name Glanochthon for Cheliderpeton latirostre after the type species Cheliderpeton vranyi had been redescribed by Werneburg and Steyer (2002) and was found not to be closely related to G. latirostre (Schoch and Witzmann, 2009b). Based on its below-demonstrated close relationship to G. latirostre, Cheliderpeton lellbachae is also referred to as Glanochthon as a new combination.
G. lellbachae co-occurs with Sclerocephalus nobilis in the same locality and horizon (Krätschmer and Resch, 2005; Schoch and Witzmann, 2009a). The combined list of autapomorphic characters 1–5 for the genus distinguish this taxon from S. nobilis and all other species of Sclerocephalus. Hypothetical larvae and small juveniles of G. lellbachae and S. nobilis may not be distinguished on the basis of the mentioned features, but S. nobilis generally has a wider jugal and more rounded orbit even at small stages. The presence of a third eryopiform temnospondyl at the Klauswald locality (suggested by Krätschmer, 2006) cannot be confirmed. With a maximum skull length of 17 cm (specimens in private collections), G. lellbachae appears to have been smaller than the more heavily ossified S. nobilis (24 cm), but admittedly the number of available specimens is very limited.
Phylogeny and taxonomy
In the analysis reported below, S. nobilis and the Glanochthon clade are found to be sister taxa. This recognized topological pattern leads to the phylogenetically problematic situation that Sclerocephalus forms a grade towards Glanochthon, which under strict application of cladistic principles would prompt erection of new genera at each node. An alternative option would be to define Sclerocephalus more broadly, encompassing all of Sclerocephalidae including Glanochthon. At the present stage, I consider any such step premature as long as morphological effects of microevolution cannot be distinguished from other effects, especially plasticity (ecophenotypes), which is beyond the scope of the present study.
Comment
Based on the current phylogenetic findings, Glanochthon is referred to as the family Sclerocephalidae, which contains a monophyletic group including a long Sclerocephalus grade and a terminal Glanochthon clade. The former referral of Glanochthon (as Cheliderpeton latirostre) within the Intasuchidae (Schoch and Milner, 2000) was based on a suite of characters which appear to be convergent in the present light of evidence.
Occurrence
Although S. nobilis and G. lellbachae co-occur in the same locality and several successive horizons, they differ in sample size per horizon. Most notably, G. lellbachae is more common in the upper fish beds (Obere Fischschiefer, “kalkige Papierschiefer”, K5), whereas S. nobilis peaks in the lower fish beds (Untere Fischschiefer, K2) as defined by Krätschmer (2004). In the other beds (K3, 4), the two taxa co-occur, mostly represented by large juveniles.
In the following description, features unique to G. lellbachae are highlighted, together with characters shared between G. lellbachae and S. nobilis, as well as G. angusta and G. latirostre. S. nobilis is consistent in most features with S. haeuseri, differing particularly in the synapomorphies shared with G. lellbachae. These differences often fall within a wide range of variation, with type specimens of G. lellbachae and S. nobilis forming end points on a continuum. Comparison with S. nobilis refer to specimens of the same size unless specifically stated otherwise because S. nobilis attained larger adult size and underwent marked ontogenetic changes in the latest phase of development (Schoch and Witzmann, 2009a). That is to say that the largest adults of G. lellbachae and S. nobilis differ even more than specimens of similar size.
4.1 Skull roof
The skull is slender with nearly straight lateral margins. Almost all skull-roofing elements are somewhat narrower than in S. nobilis. This is most conspicuous in the case of the squamosal, quadratojugal and jugal. The orbits also differ, being sagittally oval in G. lellbachae and almost perfectly round in S. nobilis. In G. lellbachae, the prefrontal and jugal form a markedly angled anterolateral region of the orbit.
The adult dermal ornament consists of elongated radial ridges, especially on the nasal, frontal, prefrontal, jugal and the anterior part of the parietal. Compared with S. nobilis, the ridges are taller and more continuous, and the polygons on the posterior skull table and cheek are larger. In the largest specimens, the ridges are tallest. Like in S. nobilis and G. angusta and in contrast to G. latirostre, lateral line sulci are entirely absent.
The tip of the snout is blunt as in S. haeuseri and in contrast to the more rounded outline in S. nobilis. The region anterior to the naris is not longer than in S. nobilis but shorter than in G. angusta. The slightly more elongated preorbital region compared to S. nobilis is dominated by a more slender and relatively longer nasal. The lacrimal is often obscured by displaced neighboring elements, especially the maxilla; it does not appear to be substantially smaller than in S. nobilis. The prefrontal is markedly distinct from that of S. nobilis; its posterior region is narrower, the anterior one is wider than in the sister taxon, and the lateral margin is nearly straight and sagittally aligned in some specimens.
In G. lellbachae, the orbits are elongate oval rather than round, and the interorbital distance (0.15–0.17) is slightly smaller than in S. nobilis (0.19–0.24), resulting from narrower postfrontals and frontals. The prefrontal–postfrontal contact is nevertheless well established. The posterior skull table is consistent with S. nobilis in the proportions and suture patterns, especially the shape of the posterior (occipital) margin of postparietals and tabulars, as well as the elongate shape of the supratemporal, which is more consistent with other Glanochthon species than Sclerocephalus species.
The cheek differs most conspicuously in some specimens with the more slender squamosal and quadratojugal bones. In G. lellbachae, the squamosal is substantially narrower than the quadratojugal, which inverses the condition in all Sclerocephalus species. The jugal is clearly narrower in the type of G. lellbachae compared with most S. nobilis specimens, but there are a range of specimens with intermediate conditions which otherwise group with either G. lellbachae or S. nobilis.
4.2 Palate
The ventral side of the skull is exposed only in SMNS 90507 (Fig. 4a, b). The palate has little to distinguish it from that of S. nobilis or any species of that genus. The vomer is wider than in other Glanochthon species, but the tusk pair is relatively larger than in S. haeuseri, which is in turn more consistent with Glanochthon. The palatine is about twice as wide anteriorly than along its posterior part and bears two large tusks aligned at the lateral margin, and there are at least two smaller teeth posterior to these. The ectopterygoid meets the palatine in an S-shaped suture, and it bears 5–6 large teeth arranged in a straight row. The palatine ramus of the pterygoid is slender and anteromedially stepped, whereas the lateral wing of the pterygoid is laterally expanded much like in Sclerocephalus species.
In the parasphenoid, the base of the cultriform process is twice as wide as the anterior two-thirds, and it bears an elongate triangular denticle field that is posteriorly continuous with that of the basal plate. The plate is wider than in G. angusta, which is more consistent with that of G. latirostre (Schoch and Witzmann, 2009b). On the basal plate, the denticle field is wedge-shaped, which is most similar to that of G. angusta. Laterally, an offset triangular region bears a marked groove, but a foramen is not preserved. Consistent with Glanochthon, and in contrast to the situation of S. haeuseri, muscular pockets along the posterolateral margin of the basal plate are not present.
4.3 Dentition
The marginal teeth are straight conical and not recurved. Their bases are striated, indicating labyrinthodont infolding of enamel and dentine. The teeth of the premaxilla and maxilla are generally smaller than those of the dentary. In the maxilla, tooth size decreases continuously towards the posterior end. The tooth count of the premaxilla is 11, that of the maxilla is unknown, and the dentary bears 21 teeth (with many irregular distances between) in UGKU POL-F 1997/1.
4.4 Braincase and occiput
There are no elements of the braincase exposed, and in the single specimen exposed in ventral view, the absence of braincase ossifications indicates that the neurocranium remained cartilaginous at least up to that stage. This is consistent with most Sclerocephalus and Glanochthon species (Boy, 1988).
4.5 Mandible
The mandible has little to distinguish it from S. haeuseri and S. nobilis, with the exception of the features described as follows. The coronoid process is somewhat more raised than in S. haeuseri. The postglenoid region is well established with a rounded posterior end and posteroventrally sloping dorsal margin. The dorsal surface is triangular and markedly concave. The postglenoid region is distinctly longer and more robust than in S. haeuseri, and its lateral side is more robustly ornamented than in other taxa.
4.6 Visceral skeleton
Only the distal, undiagnosed end of the stapes is exposed in UGKU POL-F 1997/1 (Fig. 3b). It is slender, apparently without quadrate process and with expanded distal end. No hyobranchial ossification has been identified.
4.7 Axial skeleton
The trunk is consistent in the number of vertebrae (24) with the conditions in S. nobilis and other species of Sclerocephalus and Glanochthon. However, the proportionate length of the trunk with respect to the skull is somewhat greater than in S. nobilis and about equal to that of S. haeuseri from both Jeckenbach (M6) and Pfarrwald (M9P). Furthermore, the tail (= preserved skin outline) of G. lellbachae is substantially longer than in all Sclerocephalus species, reaching the length of skull and trunk combined. The caudal skeleton ossified slowly and in adults reached only 50 % the length of the tail, as revealed by the preserved tail skin. The vertebral centra are consistent in morphology and extent of ossification with those of S. nobilis. The first four (cervical) neural arches have the same proportions and relative differences in height as in classical S. haeuseri (Boy, 1988). The anterior trunk ribs are long, and at least 6–7 of them have large blade-like uncinate processes which have blunt ends and bear large foramina presumably for blood vessels, a feature also described in large adults of S. haeuseri (Schoch and Witzmann, 2009a).
4.8 Appendicular skeleton
The dermal pectoral girdle differs in G. lellbachae in having a more slender interclavicle that is twice as long as wide. In S. nobilis, the interclavicle width usually reaches two-thirds the length, which is similar to stratigraphically older Sclerocephalus samples (Schoch and Witzmann, 2009a). In large S. nobilis and S. haeuseri, the interclavicle and clavicle are proportionately larger, and the interclavicle is even wider than in juveniles (Boy, 1988). The interclavicle has a well offset ornamented region on the ventral side with ridges most pronounced in the anterior half and a serrated anterior margin which has only half the greatest width of the element. The scapulocoracoid is larger in S. nobilis compared to G. lellbachae of similar size. This was probably simply a function of ossification, which was slightly higher in S. nobilis of the same size as G. lellbachae and much higher in adult S. nobilis, in which it reached levels nearing those of Onchiodon and Eryops (Werneburg, 2008).
There is no consistent difference in the shape and relative size of the ilium between the two taxa, although the largest adult of G. lellbachae has a markedly downcurved anteroventral margin in the acetabular region. The long axis always measures twice the length of the ventral margin, and the shaft is posterodorsally angled in adults of both taxa. The pubis remained unossified throughout life in G. lellbachae, whereas in the large adult of S. nobilis, the pubis and ischium formed a vast co-ossified plate.
The limbs are well ossified in adults (10–17 cm skull length), but even the largest specimens lack carpal ossifications. The tarsus contains three small polygonal bones arranged in an oblique row running from the fibula to the first toe (SMNS 90507). Manus and pes are slightly longer relative to skull length than they are in S. nobilis of similar size. In addition, the phalanges are markedly more slender with less broadened ends in G. lellbachae. The humerus is well differentiated with fully ossified distal condyles but no supinator. Throughout ontogeny, the humerus is substantially shorter relative to the skull (0.28–0.3 in G. lellbachae, 0.33–0.56 in S. nobilis).
The scalation of the ventral and lateral regions in the trunk is consistent with that of Sclerocephalus as described by Boy (1988) and Witzmann (2007).
5.1 Data matrix
The original data matrix contained 54 characters (Schoch and Witzmann, 2009b) to which 10 new characters were added (see Appendix A). Six taxa were added: S. sp. Concordia (from Lake Concordia deposit, St. Wendel, Quirnbach Formation; Schoch and Sobral, 2021), S. bavaricus (Boy, 1988), S. jogischneideri (Werneburg, 1992), S. nobilis (Schoch and Witzmann, 2009a), G. lellbachae and Sclerocephalus stambergi (Klembara and Steyer, 2012).
5.2 Analysis
The analysis of 64 characters and 24 taxa found a single most parsimonious tree requiring 121 steps (CI = 0.57, RI = 0.814). The analysis was conducted in the ACCTRAN mode under the New Technology search option. Bremer support values were calculated, revealing some robust support for the Glanochthon clade and the more inclusive sister group G. latirostre + G. angusta (three steps), as well as Eryopiformes in general (each three steps), a slightly lower support for Sclerocephalidae and Eryopidae (each two steps), but low support for the single nodes of the Sclerocephalus grade, as well as the relationship between archegosaurids (each just one step).
5.3 Results
In the obtained topology (Fig. 6), the general branching pattern differs from that of Schoch and Witzmann (2009b) in two major aspects: (1) Sclerocephalus forms a grade towards Glanochthon and (2) Cheliderpeton vranyi, Melosaurus and Intasuchus form successive sister taxa of the archegosaurids Archegosaurus, Platyoposaurus and Australerpeton. This is the first analysis to obtain Sclerocephalus and Glanochthon as part of the same clade rather than a grade towards stereospondyls. However, Boy (1987) was already tempted to consider Glanochthon (then only known by its stratigraphically youngest species G. latirostre) as a close relative of Sclerocephalus. The Sclerocephalidae (Sclerocephalus + Glanochthon) is thus recognized as a relatively speciose basal clade of the Stereospondylomorpha.
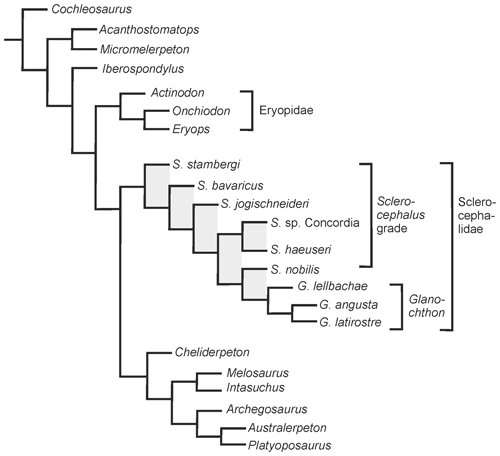
Figure 6Phylogenetic analysis of eryopiform temnospondyls with emphasis on the position of Glanochthon lellbachae.
The paraphyletic taxon Sclerocephalus was found to be a grade with respect to the genus Glanochthon, in which S. stambergi, S. bavaricus, S. jogischneideri, S. sp. Concordia + S. haeuseri and S. nobilis form successive sister taxa to Glanochthon. In the present analysis, S. haeuseri was confined to Boy's subspecies S. haeuseri haeuseri.
Sclerocephalus was the most common and regionally widespread genus in the lower Rotliegend of the Saar–Nahe Basin, and morphological change through the rock sequence was considerable (Schoch, 2009, 2014; Schoch and Witzmann, 2009a). This was already highlighted by Boy (1988) who paid tribute to the morphological change across stratigraphic levels. He formally defined S. bavaricus and S. haeuseri as chronospecies and recognized two chronosubspecies within the latter: S. haeuseri jeckenbachensis from Lake Jeckenbach (M6) and S. haeuseri haeuseri from Lake Pfarrwald (M9-P). Throughout the sequence A1–M8, there was only a single taxon present at any preserved time slice.
Before deposition of the M9 sequence, Sclerocephalus probably formed an anagenetic lineage spanning some 2 Myr (A1–Q1–M3–M6–M8) as there is no evidence of cladogenesis within the Saar–Nahe Basin. However, the phylogenetic placement of S. jogischneideri between S. bavaricus and S. sp. Concordia suggests the emigration of a post-A1 population which ultimately appeared in the Thuringian Forest (southwest Saale) Basin. This placement was already suggested by Werneburg (1992). The age of S. jogischneideri (upper Oberhof Formation) relative to that of the Saar–Nahe Basin sequence is controversial but probably not older than M9 and possibly much younger (Lützner et al., 2012).
In M9, where two different lakes existed that were separated by a tectonic structure (Kappeln M9-K in the north, Pfarrwald M9-P in the south), morphologically distinct samples appear. Whereas Lake Pfarrwald harbored classical S. haeuseri (S. haeuseri haeuseri of Boy, 1988), Lake Kappeln was inhabited by an apparently uniform population similar to S. haeuseri in the southwest (St. Wendel locality S-2 of Boy, 1987, clay pit Halseband, and road cut nearby) but two distinct taxa in the northeast (Odernheim and Alsenz regions), here referred to as S. nobilis and G. lellbachae. The latter two are likely to have diverged within the time interval between the M8 and M9 lake deposits and, as indicated by phylogenetic analysis, evolved from S. haeuseri, which is well-known from M8 deposits. These interesting patterns will be analyzed elsewhere.
Speaking within the framework of Boy's chronospecies concept, the ancestor of S. nobilis and G. lellbachae branched off after S. h. jeckenbachensis and before S. h. haeuseri. As Boy (1988) noted, S. h. haeuseri had evolved a narrow interorbital region and slender posterior skull table, which was considered a derived character of later S. haeuseri (Schoch et al., 2019). However, these features are unique to the population in Lake Pfarrwald, which falls within M9 rather than M10 as originally considered by Boy (1988), a fact that became only apparent after more research had been conducted (Boy et al., 2012). The subsequent, true M10 population of S. haeuseri from the lowermost horizons of Lake Humberg (Odernheim region) differs from the M9-P Pfarrwald population (S. h. haeuseri), instead sharing more aquatic features with M8 S. haeuseri (Schoch, 2009). Hence, Pfarrwald S. haeuseri is more likely to form a regionally isolated population, whereas M10 S. haeuseri more likely evolved from a population in Lake Kappeln, such as the one preserved in the M9-K sample from St. Wendel.
A more detailed assessment of the relationships between all species referred to Sclerocephalus will be carried out elsewhere. In the present cladogram, phylogenetic positioning might be hypothesized as being influenced by size disparity and ontogenetic disparity, with S. stambergi and S. jogischneideri both found to be basal and relatively small taxa. In contrast to the rather adult morphology of S. jogischneideri, S. stambergi has an immature appearance, probably representing a juvenile, by analogy with the ontogenetically well-sampled S. haeuseri (Boy, 1988). Furthermore, both S. jogischneideri and S. stambergi are known from a single specimen each, whereas the other taxa or samples (“populations”) are represented by dozens or sometimes hundreds of specimens (Schoch, 2009; Krätschmer, 2004), and some stratigraphically (and probably phylogenetically) younger samples (Lake Odernheim, M8; Lake Humberg, M10) are known by small, paedomorphic adults only (Schoch, 2009). Furthermore, as large adults are very rare, they are often found only after many years of continued collecting. Admittedly, this fact also weakens the size disparity between S. nobilis and G. lellbachae mentioned above.
Phylogenetic analysis indicates that G. lellbachae forms the stratigraphically oldest taxon of the Glanochthon clade, sharing a range of synapomorphies with its stratigraphically younger relatives G. angusta (M10c) and G. latirostre (M10d). The three taxa might well form an anagenetic lineage, but speciation events cannot be ruled out. The two different hypotheses can only be tested by more detailed geographic and stratigraphic sampling, which is hardly possible without numerous new additional outcrops.
A new, still to be formally named species of Glanochthon was reported by Steyer (1996) from Buxières-les-Mines (Allier basins, France). Boy and Schindler (2012) and Schneider and Werneburg (2012) concur that the corresponding rock unit, the Membre supérieur of the Assise de Buxières-Autunien gris (Steyer et al., 2000), falls within the uppermost part of the Meisenheim Formation. This is consistent with the proposed hypothesis of an origin of Glanochthon within the Saar–Nahe Basin and a subsequent emigration into the Allier basins.
The current phylogenetic findings are not readily translated into a taxonomic scheme. The easiest solution would be to grant each sample from a different lake deposit a separate species name. This is practiced here in the straightforward case of Glanochthon, but in the vast series of samples of Sclerocephalus haeuseri, Boy's (1988) chronosubspecies approach still remains more appealing. A morphometric study analyzing this interesting problem is under way and will be published elsewhere. Provided that the findings of the present study are correct, Sclerocephalus forms a paraphyletic assemblage with respect to the Glanochthon clade. The logical and phylogenetically correct approach would be to erect new genera for the successive species, but the close resemblance of most of these taxa would make these difficult to define. The extraordinary detailed stratigraphic and morphological record of the Sclerocephalus–Glanochthon clade therefore demonstrates the limits of any taxonomic approach to classify evolving lineages.
| 1. | Premaxilla (alary process). Absent (0) or present (1). |
| 2. | Premaxilla (prenarial portion). Short (0) or expanded anteriorly by about the length of the naris (1). |
| 3. | Premaxilla (outline). Parabolically rounded (0) or box-like, anteriorly blunt (1). |
| 4. | Snout (internarial distance). Narrower than interorbital distance (0) or wider (1). |
| 5. | Snout (margin). Straight (0) or laterally constricted at level of naris (1). |
| 6. | Rostrum. Absent (0) or present (1). |
| 7. | Internarial fenestra. Absent (0) or present (1). |
| 8. | Orbits. Round to slightly oval (0) or elongated oval (1). |
| 9. | Orbits. Ends rounded (0) or pointed (1). |
| 10. | Maxilla (anterior margin). Straight (0) or laterally convex due to enlarged teeth (1). |
| 11. | Maxilla (contact to nasal). Absent, separated by lacrimal (0) or present (1). |
| 12. | Nasal (lateral margin). Straight (0) or stepped with lateral excursion anterior to prefrontal, accommodating narrower lacrimal (1). |
| 13. | Lacrimal (length). As long as nasal (0), shorter than nasal (1) or much abbreviated (2). |
| 14. | Lacrimal (width). Lateral suture parallels medial one (0) or lateral suture posterolaterally expanded to give broader preorbital region (1). |
| 15. | Preorbital region (length). Less than twice the length of posterior skull table (0) or more (1). |
| 16. | Prefrontal-jugal (contact). Absent (0) or present (1). |
| 17. | Prefrontal (anterior end). Pointed (0) or wide and blunt (1). |
| 18. | Frontal-nasal (length). Frontal as long or longer than nasal (0) or shorter (1). |
| 19. | Interorbital distance. Narrower than orbital width (0) or wider (1). |
| 20. | Lateral line (sulci). Absent in adults (0) or present (1). |
| 21. | Posterior skull table (length). Less than 0.7 times the width (0), 0.7–0.8 times (1) or larger than 0.8 (2). |
| 22. | Intertemporal. Present (0) or absent (1). |
| 23. | Postorbital. Long triangular, wedged deeply between squamosal and supratemporal (0) or short (1). |
| 24. | Squamosal embayment (size). Wide, giving semilunar flange on squamosal (0) or slit-like with thin flange on squamosal (1). |
| 25. | Tabular (ventral crest). Absent (0) or present (1). |
| 26. | Jugal (preorbital expansion). Absent in adults (0) or present (1). |
| 27. | Ornament. Polygons and short ridges (0) or long ridges arranged radially (1). |
| 28. | Vomer. Smooth (0) or with paired depressions anteriorly (1). |
| 29. | Vomerine tusks. Anterolateral to choana, transverse row (0) or well anterior to choana, sagittal row (1). |
| 30. | Anterior palatal openings. Absent (0) or present (1). |
| 31. | Choana (width). Elongated oval or slit-like (0) or round (1). |
| 32. | Premaxilla. Borders choana (0) or not (1). |
| 33. | Palatine, ectopterygoid (continuous tooth row). Absent (0) or present (1). |
| 34. | Palatine. Fangs and no more than 3–4 extra teeth (0) or 5 or more extra teeth (1). |
| 35. | Ectopterygoid (tusks). Present (0) or absent (1). |
| 36. | Parasphenoid. Denticle field on plate triangular (0) or round (1). |
| 37. | Basipterygoid ramus (length). Transverse, rod-like (0) or short without medial extension (1). |
| 38. | Basicranial articulation. Moveable overlap (0) or tightly sutured (1). |
| 39. | Carotid foramina (entrance). Anteromedial on basal plate, close to cultriform process (0) or at posterolateral corner of plate (1). |
| 40. | Vomer. Separated by pterygoid from interpterygoid vacuity (0) or bordering that opening (1). |
| 41. | Cultriform process (width). Throughout of similar width (0) or posteriorly expanding abruptly to about twice the width (1). |
| 42. | Stapes (quadrate process). Absent (0) or present (1). |
| 43. | Interclavicle (adult shape). As wide as long (0) or longer than wide (1). |
| 44. | Interclavicle (width). As wide or wider than posterior skull table (0) or narrower (1). |
| 45. | Interclavicle (size). Shorter than posterior skull table (0) or longer than half of skull length (1). |
| 46. | Interclavicle (posterior margin). Triangular, pointed (0) or rounded to blunt (1). |
| 47. | Interclavicle (outline). Rhomboid (0) or quadrangular to pentagonal (1). |
| 48. | Humerus (entepicondylar foramen). Present (0) or absent (1). |
| 49. | Humerus (supinator). Present (0) or absent (1). |
| 50. | Humerus. Short with slow growth rate in larvae (0) or long due to rapid growth (1). |
| 51. | Femur. Intercondylar fossa on dorsodistal surface forming deep trough (0) or shallow groove (1). |
| 52. | Pubis. Ossified (0) or unossified (1). |
| 53. | Ilium. Shaft kinked, posteriorly directed (0), shaft straight and dorsal with broadened end (1), or shaft straight posterodorsally directed (2). Unordered. |
| 54. | Ribs. Short (0), long rod-like with small uncinates (1) or long with blade-like uncinates (2). Unordered. |
| 55. | Interpterygoid vacuities. Longer than vomer and premaxilla (0) or equal to or shorter (1). |
| 56. | Neurocranium. Cartilaginous or only partially ossified (0) or fully ossified with sphenoid and ethmoid portions (1). |
| 57. | Squamosal embayment. Framed by parallel squamosal and tabular margins (0) or forming medially rounded extension, constricting the posterior skull table (1). |
| 58. | Supratemporal. Less than or about 2 times longer than wide (0) or more than 2 times longer than wide (1). |
| 59. | Squamosal. Posterior part as wide as quadratojugal (0) or markedly narrower (1). |
| 60. | Jugal. Wider than orbit (0) or markedly narrower (1). |
| 61. | Premaxilla. Lateral margin straight (0) or bulging laterally (1). |
| 62. | Snout. Shorter than 2 times the length of postorbital skull table (0) or as long as or longer (1). |
| 63. | Lacrimal. At least two-thirds the length of the preorbital skull (0) or shorter (1). |
| 64. | Quadrate. Wedging in between quadratojugal and squamosal posteriorly (0) or offset from the posterior margin of the dermal cheek bones (1). |
| Dendrysekos_helogenes |
| 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 ? 0 0 0 0 0 0 0 0 0 1 0 0 0 0 |
| Balanerpeton_woodi |
| 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 |
| Cochleosaurus_bohemicus |
| 0 1 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 |
| Micromelerpeton_credneri |
| 1 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 1 1 1 1 1 0 0 1 0 1 0 0 0 0 1 0 0 0 0 |
| Acanthostomatops_vorax |
| 1 1 0 0 0 0 1 0 0 0 0 0 0 0 1 0 0 0 1 0 0 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 1 1 1 0 1 0 1 2 0 1 0 0 0 0 0 0 0 0 0 |
| Iberospondylus_schultzei |
| 1 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 ? ? 0 0 ? ? ? ? ? ? ? ? ? ? ? 1 1 0 0 0 0 0 0 0 0 0 |
| Eryops_megacephalus |
| 1 0 1 1 0 0 0 0 0 1 0 0 0 1 1 1 1 1 1 0 0 1 1 1 0 1 0 1 0 0 1 0 0 0 0 0 0 1 1 1 1 0 0 0 0 1 0 1 0 0 1 0 2 2 0 1 0 0 0 0 0 0 1 0 |
| Onchiodon_labyrinthicus |
| 1 0 1 1 0 0 0 0 0 1 0 0 0 1 1 1 1 0 1 0 0 1 1 1 0 1 0 1 0 0 1 0 0 0 0 0 0 1 1 1 1 0 0 0 ? 1 0 1 0 0 1 0 2 2 0 0 0 0 0 0 0 0 0 0 |
| Actinodon_frossardi |
| 1 0 1 1 0 0 0 0 0 0 0 0 0 0 1 1 0 0 1 0 0 1 0 1 0 1 0 1 0 0 0 0 0 0 0 0 0 1 1 1 1 ? 0 0 1 1 0 1 0 ? 1 0 ? 2 0 0 0 0 0 0 0 0 0 0 |
| “Sclerocephalus”_stambergi |
| 1 0 1 1 0 0 0 0 0 0 1 1 1 0 0 1 0 0 0 ? 2 1 0 1 1 1 0 ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? 1 1 1 ? ? ? ? ? ? ? ? ? ? ? 0 0 0 0 0 0 0 0 |
| Sclerocephalus_bavaricus |
| 1 1 0 1 0 0 0 0 0 0 1 1 2 0 0 1 0 0 0 1 2 1 0 1 1 1 0 1 0 0 0 0 ? 0 0 0 0 0 1 1 0 ? 1 0 1 0 0 1 ? 0 1 ? 1 2 ? ? 0 0 0 0 0 0 1 1 |
| Sclerocephalus_haeuseri |
| 1 0 1 1 0 0 0 0 0 1 1 2 2 0 0 1 0 0 0 0 2 1 0 1 1 1 0 1 0 0 0 0 1 0 0 0 0 0 1 1 0 1 1 0 1 0 0 1 0 0 1 0 1 2 1 0 1 0 0 0 0 0 1 1 |
| Sclerocephalus_sp._Concordia |
| 1 0 1 1 0 0 0 0 0 1 1 2 2 0 0 1 0 0 0 0 2 1 0 1 1 1 0 1 0 0 0 0 1 0 0 0 0 1 1 1 0 1 1 0 1 0 0 1 0 0 1 ? 1 2 0 1 0 0 0 0 0 0 1 1 |
| Sclerocephalus_jogischneideri |
| 1 0 1 1 0 0 0 0 0 1 1 2 2 0 0 1 0 0 0 1 2 1 0 1 1 1 0 1 0 0 0 0 1 0 0 0 0 0 1 1 0 ? 1 0 1 0 0 1 ? 0 1 ? 1 2 1 0 0 0 0 0 0 0 1 1 |
| Sclerocephalus_nobilis |
| 1 1 1 1 0 0 0 0 0 1 1 2 1 0 0 1 0 0 0 0 2 1 0 1 1 1 0 1 0 0 0 0 1 0 0 0 0 0 1 1 0 ? 1 0 1 0 0 1 0 0 1 0 1 2 1 0 1 1 1 0 0 1 1 1 |
| Glanochthon_lellbachae |
| 1 1 1 1 1 0 0 0 1 1 1 1 ? 0 1 1 0 0 0 1 2 1 0 1 1 1 0 1 0 0 0 0 1 0 1 0 0 0 1 1 0 0 1 1 1 0 0 1 1 0 1 0 1 1 1 0 0 1 1 1 0 1 1 1 |
| Glanochthon_angusta |
| 1 1 1 1 1 0 0 0 1 1 1 1 ? 0 1 1 0 0 0 1 2 1 0 1 1 1 0 1 0 0 0 0 1 0 1 0 0 0 1 1 0 0 1 1 1 0 0 1 1 0 1 1 1 1 1 0 0 1 1 1 1 1 1 1 |
| Glanochthon_latirostre |
| 1 1 1 1 1 0 0 0 1 1 1 1 1 0 1 1 0 0 0 1 1 1 0 1 1 1 1 1 0 0 0 0 1 0 1 0 0 0 1 1 0 0 1 1 1 0 0 1 1 0 1 1 1 1 1 0 0 1 1 1 1 1 1 1 |
| Intasuchus_silvicola |
| 1 1 0 1 0 0 0 1 ? 0 0 0 0 0 1 1 0 1 0 ? 0 1 0 1 ? 1 1 1 0 0 0 1 1 1 1 0 1 0 1 1 0 ? ? ? ? ? ? ? ? ? ? ? ? ? 1 0 0 0 0 0 0 0 0 0 |
| Melosaurus_uralensis |
| 1 1 0 1 0 0 0 1 0 0 1 0 0 0 1 1 0 1 0 ? 1 1 0 1 ? 1 0 ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? 1 1 ? 0 ? ? 1 ? ? ? ? ? 1 0 0 0 0 0 0 0 0 0 |
| Cheliderpeton_vranyi |
| 1 0 0 1 0 0 0 0 0 0 ? 0 0 0 0 1 0 1 0 1 2 1 0 1 ? 1 0 ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? 1 0 1 0 0 1 1 0 1 1 2 1 1 0 0 0 0 0 0 0 0 0 |
| Archegosaurus_decheni |
| 1 1 0 1 0 1 0 1 0 0 1 0 0 0 1 1 0 1 0 1 2 1 0 1 1 1 1 1 1 0 0 1 1 1 1 1 1 0 1 1 0 1 1 1 1 0 0 1 1 0 1 1 1 1 1 0 0 0 0 1 0 0 0 0 |
| Platyoposaurus_stuckenbergensis |
| 1 1 0 1 0 1 0 0 0 0 1 0 0 0 1 1 0 1 0 1 2 1 0 1 1 1 0 1 1 1 0 1 1 1 1 1 1 0 1 1 0 0 1 1 1 0 0 1 1 ? 1 1 1 1 1 0 0 0 0 0 0 0 0 0 |
| Australerpeton_cosgriffi |
| 1 1 0 1 0 1 0 0 0 0 1 0 0 0 1 1 0 1 0 1 2 1 0 1 1 1 0 1 1 1 0 1 1 1 1 1 1 1 1 1 0 0 1 1 1 0 0 1 0 ? 1 1 1 1 1 0 0 0 0 0 0 0 0 0 |
Software package available under: https://cladistics.org/tnt (Willi Hennig Society, 2021).
All underlying research data are provided in the Appendices A and B.
The author declares that there is no conflict of interest.
I thank Isabell Rosin (Stuttgart) for skillfully preparing original material and producing casts, Manuela Aiglstorfer (Mainz), Edgar Müller (Landsweiler-Reden), and Sebastian Voigt (Thallichtenberg) for access to material, and Klaus Krätschmer (Odernheim), Andrew Milner (London), Ralf Werneburg (Schleusingen), and Florian Witzmann (Berlin) for many helpful discussions. I thank Bryan Gee, Andrew Milner, and Marcello Ruta for their very helpful reviews.
This paper was edited by Florian Witzmann and reviewed by Andrew Milner, Marcello Ruta, and Bryan Gee.
Boy, J. A.: Die Tetrapoden-Lokalitäten des saarpfälzischen Rotliegenden (?Ober-Karbon-Unter-Perm; SW-Deutschland) und die Biostratigraphie der Rotliegend-Tetrapoden, Mainzer geowiss. Mitt., 16, 31–65, 1987.
Boy, J. A.: Über einige Vertreter der Eryopoidea (Amphibia: Temnospondyli). 1. Sclerocephalus, Paläontol. Z., 62, 429–457, 1988.
Boy, J. A.: Über einige Vertreter der Eryopoidea (Amphibia: Temnospondyli). 4. Cheliderpeton latirostre, Paläontol. Z., 67, 123–143, 1993.
Boy, J. A.: Seen der Rotliegend-Zeit – ein Lebensraum vor rund 300 Millionen Jahren in der Pfalz, in: Erdgeschichte im Rheinland, edited by: Koenigswald, W., Friedrich Pfeil, Munich, 107–116, 1994.
Boy, J. A.: Ein neuer Eryopoide (Amphibia: Temnospondyli) aus dem saarpfälzischen Rotliegend (Unter-Perm; SW-Deutschland), Mainzer geowiss. Mitt., 25, 7–26, 1996.
Boy, J. A.: Paläoökologische Rekonstruktion von Wirbeltieren: Möglichkeiten und Grenzen, Paläontol. Z., 77, 123–152, 2003.
Boy, J. A. and Schindler, T.: Ökostratigraphie des Rotliegend, in: Stratigraphie von Deutschland X. Rotliegend Teil I: Innervariscische Becken, edited by: Lützner, H. and Kowalczyk, G., Schweizerbart, Stuttgart, 143–160, 2012.
Boy, J. A., Haneke, J., Kowalczyk, G., Lorenz, V., Schindler, T., Stollhofen, H., and Thum, H.: Rotliegend im Saar-Nahe-Becken, am Taunus-Südrand und im nördlichen Oberrheingraben, in: Stratigraphie von Deutschland X. Rotliegend Teil I: Innervariscische Becken, edited by: Lützner, H. and Kowalczyk, G., Schweizerbart, Stuttgart, 254–377, 2012.
Jordan, H.: Ergänzende Beobachtungen zu der Abhandlung von Goldfuss über die Gattung Archegosaurus mit einer Anmerkung von F. Müller, Verh. Naturhist. Ver. Rheinl., 6, 67–81, 1849.
Klembara, J. and Steyer, J. S.: A new species of Sclerocephalus (Temnospondyli: Stereospondylomorpha) from the Early Permian of the Boskovice Basin (Czech Republic), J. Paleontol., 86, 302–310, 2012.
Krätschmer, K.: Revision von “Sclerocephalus haeuseri” (Goldfuss) 1847 (Stem-Stereospondyli), Geowiss. Beitr. saarpfälz. Rotl., 2, 1–52, 2004.
Krätschmer, K.: Neue temnospondyle Amphibien aus dem Rotliegend des südwestdeutschen Saar-Nahe-Becken. Teil 1, Geowiss. Beitr. saarpfälz. Rotl., 4, 3–46, 2006.
Krätschmer, K. and Resch, M.: Klauswaldia nobile gen. et spec. nov. Ein außergewöhnlicher stereospondyler Stegocephale aus dem Rotliegend (Unter-Perm) von Odernheim (Rheinland-Pfalz, SW-Deutschland), Geowiss. Beitr. saarpfälz. Rotl., 3, 39–65, 2005.
Kriwet, J., Witzmann, F., Klug, S., and Heidtke, U. H. J.: First evidence of a vertebrate three-level trophic chain in the fossil record, P. Roy. Soc. B., 275, 181–186, 2008.
Lützner, H., Kowalczyk, G., and Schneider, J. W.: Stratigraphische Korrelation der innervariscischen Rotliegendbecken in Deutschland, in: Stratigraphie von Deutschland X. Rotliegend Teil I: Innervariscische Becken, edited by: Lützner, H. and Kowalczyk, G., Schweizerbart, Stuttgart, 861–879, 2012.
Menning, M. Bachtadse, V.: Magnetostratigraphie und globale Korrelation des Rotliegend innervariscischer Becken, in: Stratigraphie von Deutschland X. Rotliegend Teil I: Innervariscische Becken, edited by: Lützner, H. and Kowalczyk, G., Schweizerbart, Stuttgart, 176–203, 2012.
Schneider, J. and Werneburg, R.: Biostratigraphie des Rotliegend mit Insekten und Amphibien, Schriftenreihe Deut. Ges. Geowiss., 61, 110–142, 2012.
Schoch, R. R.: Life-cycle evolution as a response to diverse lake habitats in Paleozoic amphibians, Evolution, 63, 2738–2749, 2009.
Schoch, R. R.: The major clades of temnospondyls: an inclusive phylogenetic analysis, J. Syst. Palaeontol., 11, 673–705, 2013.
Schoch, R. R.: Amphibian Evolution. The Life of Early Land Vertebrates, Wiley, Chichester, UK, 264 pp., 2014.
Schoch, R. R. and Hampe, O.: An eryopid-like temnospondyl from the Lower Rotliegend Meisenheim Formation of the Saar-Nahe Basin, Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 232, 315–323, 2004.
Schoch, R. R. and Milner, A. R.: Stereospondyli, in: Handbuch der Paläoherpetologie, Vol. 3B,, edited by: Wellnhofer, P., Friedrich Pfeil, Munich, 203 pp., 2000.
Schoch, R. R. and Sobral, G.: A new species of Sclerocephalus with a fully ossified endocranium gives insight into braincase evolution in temnospondyls, J. Vertebr. Palaeontol., in press, 2021.
Schoch, R. R. and Witzmann, F.: Osteology and relationships of the temnospondyl genus Sclerocephalus, Zool. J. Linn. Soc.-Lond., 157, 135–168, 2009a.
Schoch, R. R. and Witzmann, F.: The temnospondyl Glanochthon from the Lower Permian Meisenheim Formation of Germany, Spec. Pap. Palaeont., 81, 121–136, 2009b.
Schoch, R. R., Ebert, M., and Robert, E.: Type specimen of Sclerocephalus haeuseri Goldfuss, 1847 rediscovered, N. Jb. Geol. Paläont. Abh., 292, 315–320, 2019.
Steyer, J. S.: Une nouvelle espèce de Cheliderpeton (Amphibia, Temnospondyli) due Permien inférieur de Buxières-les-Mines (Allier, France). Position phylétique et relations ontogénie-phylogénie des eryopides, Diploma Thesis, Academie de Montpellier, Université Montpellier II Sciences et techniques du Langedoc, 31 pp., 1996.
Steyer, J. S., Escuille, F., Pouillon, J. M., Broutin, J., Debriette, P., Freytet, P., Gand, G., Poplin, C., Rage, J. C., Rival, J., Schneider, J. W., Stamberg, S., Werneburg, R., and Cuny, G.: New data on the flora and fauna from the ?uppermost Carboniferous-Lower Permian of Buxières-les-Mines, Bourbon l'Archambault Basin (Allier, France). A preliminary report, B. Soc. Géol. Fr., 171, 239–249, 2000.
Watson, D. M. S.: The structure, evolution, and origin of the Amphibia. The orders Rachitomi [sic] and Stereospondyli, Philos. T. Roy. Soc. B, 209, 1–73, 2019.
Werneburg, R.: Sclerocephalus jogischneideri n. sp. (Eryopoidea, Amphibia) aus dem Unterrotliegenden (Unterperm) des Thüringer Waldes, Freiberger Forschungsh., C 445, 29–48, 1992.
Werneburg, R.: Der „Manebacher Saurier“ – ein neuer großer Eryopide (Onchiodon) aus dem Rotliegend (Unter-Perm) des Thüringer Waldes, Veröff. Naturhist. Mus. Schleusingen, 22, 3–40, 2008.
Werneburg, R. and Steyer, J. S.: Revision of Cheliderpeton vranyi Fritsch, 1877 (Amphibia, Temnospondyli) from the Lower Permian of Bohemia (Czech Republic), Paläontol. Z., 76, 149–162, 2002.
Willi Hennig Society: Tree Analysis Technology, available at: https://cladistics.org/tnt, last access: 19 March 2021.
Witzmann, F.: The evolution of the scalation pattern in temnospondyl amphibians, Zool. J. Linn. Soc.-Lond., 150, 815–834, 2007.
Zittel, K. A. v.: Handbuch der Palaeontologie: I. Abtheilung. Palaeozoologie. III. Band. Vertebrata (Pisces, Amphibia, Reptilia, Aves), Berlin, 900 pp., 1888.